当前位置:
X-MOL 学术
›
Adv. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zwitterions and betaines as highly soluble materials for sustainable corrosion protection: Interfacial chemistry and bonding with metal surfaces
Advances in Colloid and Interface Science ( IF 15.9 ) Pub Date : 2024-01-23 , DOI: 10.1016/j.cis.2024.103091 Chandrabhan Verma 1 , Shikha Dubey 2 , Ranjith Bose 1 , Akram Alfantazi 1 , Eno E Ebenso 3 , Kyong Yop Rhee 4
Advances in Colloid and Interface Science ( IF 15.9 ) Pub Date : 2024-01-23 , DOI: 10.1016/j.cis.2024.103091 Chandrabhan Verma 1 , Shikha Dubey 2 , Ranjith Bose 1 , Akram Alfantazi 1 , Eno E Ebenso 3 , Kyong Yop Rhee 4
Affiliation
|
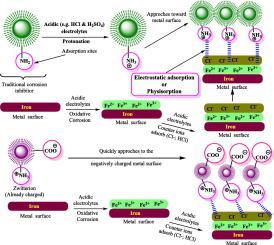
The primary requirements for interfacial adsorption and corrosion inhibition are solubility and the existence of polar functional groups, particularly charges. Traditional organic inhibitors have a solubility issue due to the hydrophobic moieties they incorporate. Most documented organic inhibitors have aromatic rings, hydrocarbon chains, and a few functional groups. The excellent solubility and high efficacy of zwitterions and betaines make them the perfect replacements for insoluble corrosion inhibitors. Zwitterions and betaines are more easily soluble because of interactions between their positive and negative charges (–COO, –PO, –NH, –NHR, –NHR, –SO etc.) and the polar solvents. The positive and negative charges also aid these molecules' physical and chemical adsorption at the metal-electrolyte interfaces. They develop a corrosion-inhibiting layer through their adsorption. After becoming adsorbed at the metal-electrolyte interface, they act as mixed-type inhibitors, slowing both cathodic and anodic processes. They usually adsorb according to the Langmuir adsorption isotherm. In this article, the corrosion inhibition potential of zwitterions and betaines in the aqueous phase, as well as their mode of action, are reviewed. This article details the advantages and disadvantages of utilizing zwitterions and betaines for sustainable corrosion protection.
中文翻译:

两性离子和甜菜碱作为可持续腐蚀保护的高溶解性材料:界面化学和与金属表面的粘合
界面吸附和腐蚀抑制的主要要求是溶解度和极性官能团(特别是电荷)的存在。传统的有机抑制剂由于含有疏水性部分而存在溶解度问题。大多数记录的有机抑制剂都具有芳香环、烃链和一些官能团。两性离子和甜菜碱的优异溶解度和高效性使其成为不溶性缓蚀剂的完美替代品。两性离子和甜菜碱更容易溶解,因为它们的正电荷和负电荷(-COO、-PO、-NH、-NHR、-NHR、-SO 等)与极性溶剂之间存在相互作用。正电荷和负电荷还有助于这些分子在金属-电解质界面处的物理和化学吸附。它们通过吸附形成腐蚀抑制层。吸附在金属-电解质界面后,它们充当混合型抑制剂,减缓阴极和阳极过程。它们通常根据朗缪尔吸附等温线进行吸附。本文综述了两性离子和甜菜碱在水相中的缓蚀潜力及其作用方式。本文详细介绍了利用两性离子和甜菜碱进行可持续腐蚀防护的优点和缺点。
更新日期:2024-01-23
中文翻译:

两性离子和甜菜碱作为可持续腐蚀保护的高溶解性材料:界面化学和与金属表面的粘合
界面吸附和腐蚀抑制的主要要求是溶解度和极性官能团(特别是电荷)的存在。传统的有机抑制剂由于含有疏水性部分而存在溶解度问题。大多数记录的有机抑制剂都具有芳香环、烃链和一些官能团。两性离子和甜菜碱的优异溶解度和高效性使其成为不溶性缓蚀剂的完美替代品。两性离子和甜菜碱更容易溶解,因为它们的正电荷和负电荷(-COO、-PO、-NH、-NHR、-NHR、-SO 等)与极性溶剂之间存在相互作用。正电荷和负电荷还有助于这些分子在金属-电解质界面处的物理和化学吸附。它们通过吸附形成腐蚀抑制层。吸附在金属-电解质界面后,它们充当混合型抑制剂,减缓阴极和阳极过程。它们通常根据朗缪尔吸附等温线进行吸附。本文综述了两性离子和甜菜碱在水相中的缓蚀潜力及其作用方式。本文详细介绍了利用两性离子和甜菜碱进行可持续腐蚀防护的优点和缺点。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号