当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of electrochemical carbon dioxide reduction to formate on tin electrode
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-01-22 , DOI: 10.1016/j.cej.2024.148972
Anoop Naikkath , Nikhil George Mohan , Kothandaraman Ramanujam , Ramanathan Srinivasan
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-01-22 , DOI: 10.1016/j.cej.2024.148972
Anoop Naikkath , Nikhil George Mohan , Kothandaraman Ramanujam , Ramanathan Srinivasan
|
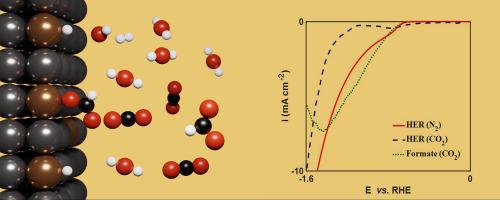
Carbon dioxide can be electrochemically reduced to formate on tin electrodes. Formate is one of the most economically viable products of CO2 reduction reaction (CO2 RR). While CO2 is reduced to formate, hydrogen evolution reaction (HER) occurs concomitantly. The kinetics of CO2 RR and HER on the tin electrode was investigated. Potentiodynamic polarization studies were conducted in CO2 saturated and N2 saturated 0.1 M KHCO3 solutions, in the potential range from −0.014 V to −1.59 V vs. RHE. A faradaic efficiency of 93.95 % towards formate production was obtained at −1.09 V vs. RHE. The mass transfer effects and bicarbonate dissociation equilibrium were used to estimate the concentration of the reactants at the electrode surface. Density functional theory calculations indicate that –OCHO intermediate species is thermodynamically favoured, and a four-step reaction with two intermediates is proposed. The proposed mechanism captures the major features of the polarization data. CO2 reduction occurs via two intermediate species, viz. adsorbed H and OCHO species, while HER occurs via Volmer-Heyrovsky steps. The model predicts that in N2 saturated solutions, the fractional surface coverage of adsorbed H reaches a maximum of ∼0.48 at a potential of −0.82 V vs. RHE while in CO2 saturated solutions, the corresponding value is ∼0.29. In addition, the maximum fractional surface coverage of adsorbed OCHO is predicted to be ∼0.12 in CO2 saturated solutions.
中文翻译:

锡电极电化学还原二氧化碳生成甲酸盐的机理
二氧化碳可以在锡电极上电化学还原生成甲酸盐。甲酸是二氧化碳还原反应 (CO2RR) 中最经济可行的产物之一。当CO2被还原成甲酸盐时,同时发生析氢反应(HER)。研究了锡电极上 CO2RR 和 HER 的动力学。动电位极化研究在 CO2 饱和和 N2 饱和 0.1 M KHCO3 溶液中进行,电位范围为 -0.014 V 至 -1.59 V vs. RHE。在-1.09 V vs. RHE 下获得了 93.95% 的甲酸生产法拉第效率。传质效应和碳酸氢盐解离平衡用于估计电极表面反应物的浓度。密度泛函理论计算表明,-OCHO 中间体物种在热力学上是有利的,并且提出了两种中间体的四步反应。所提出的机制捕获了偏振数据的主要特征。 CO2 减少是通过两种中间物质发生的,即。吸附 H 和 OCHO 物质,而 HER 通过 Volmer-Heyrovsky 步骤发生。该模型预测,在 N2 饱和溶液中,吸附 H 的表面覆盖分数在电位为 -0.82 V vs. RHE 时达到最大值 ∼0.48,而在 CO2 饱和溶液中,相应值为 ∼0.29。此外,在 CO2 饱和溶液中,吸附 OCHO 的最大表面覆盖率预计为 ∼0.12。
更新日期:2024-01-22
中文翻译:

锡电极电化学还原二氧化碳生成甲酸盐的机理
二氧化碳可以在锡电极上电化学还原生成甲酸盐。甲酸是二氧化碳还原反应 (CO2RR) 中最经济可行的产物之一。当CO2被还原成甲酸盐时,同时发生析氢反应(HER)。研究了锡电极上 CO2RR 和 HER 的动力学。动电位极化研究在 CO2 饱和和 N2 饱和 0.1 M KHCO3 溶液中进行,电位范围为 -0.014 V 至 -1.59 V vs. RHE。在-1.09 V vs. RHE 下获得了 93.95% 的甲酸生产法拉第效率。传质效应和碳酸氢盐解离平衡用于估计电极表面反应物的浓度。密度泛函理论计算表明,-OCHO 中间体物种在热力学上是有利的,并且提出了两种中间体的四步反应。所提出的机制捕获了偏振数据的主要特征。 CO2 减少是通过两种中间物质发生的,即。吸附 H 和 OCHO 物质,而 HER 通过 Volmer-Heyrovsky 步骤发生。该模型预测,在 N2 饱和溶液中,吸附 H 的表面覆盖分数在电位为 -0.82 V vs. RHE 时达到最大值 ∼0.48,而在 CO2 饱和溶液中,相应值为 ∼0.29。此外,在 CO2 饱和溶液中,吸附 OCHO 的最大表面覆盖率预计为 ∼0.12。

































 京公网安备 11010802027423号
京公网安备 11010802027423号