当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The non-radical, non-ROS, and non-Cu(III) oxidation of inert pollutants significantly enhanced by CuO-MgO composites
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-01-22 , DOI: 10.1016/j.cej.2024.149003
Ning Jiang , Lihong Wang , Haorui Wang , Meng Jiao , Tao Zhang
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-01-22 , DOI: 10.1016/j.cej.2024.149003
Ning Jiang , Lihong Wang , Haorui Wang , Meng Jiao , Tao Zhang
|
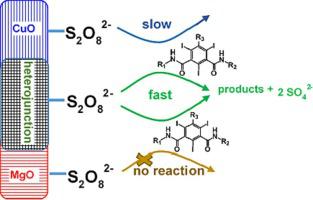
Peroxydisulfate (PDS) is an appealing oxidant to eliminate water pollutants due to the relatively low cost and high stability compared with other peroxides. It needs to be activated for application, with non-radical producing processes being specifically preferred to obtain high selectivity and to reduce useless consumption of active species by ubiquitous chloride ions. Unfortunately, most of the current non-radical oxidation processes of PDS are ineffective toward inert pollutants. Here we report a novel non-radical PDS oxidation process initiated by CuO-MgO composites toward effective removing refractory iodinated contrast media compounds (ICMs). The optimized CuO-MgO composite was 18-times more efficient than CuO in activating PDS to remove iopamidol, a typical refractory ICM, and exhibited much lower Cu2+ leaching. Interestingly, the enhanced oxidation is not related to radicals, reactive oxygen species (ROS), or high valent Cu(III). We revealed with a series of surface characterization and density functional theory calculation that the CuO-MgO composite has Cu2+ -Mg2+ heterojunctions of high electron density, thus is more active than CuO alone for PDS activation. PDS interacted with the composite surface in inner-sphere mode; consequently, the surface-bound PDS abstracted electrons directly from inert pollutants. Due to the lower oxidation power than traditional radicals, PDS/CuO-MgO oxidation of the ICM probe was insensitive to chloride and produced less hydroxyl- and nitro- degradation products. This research highlights that Cu2+ -Mg2+ heterojunctions can play an important role in enhancing the non-radical and non-ROS oxidation capability of PDS toward inert pollutants.
中文翻译:

CuO-MgO 复合材料显着增强了惰性污染物的非自由基、非 ROS 和非 Cu(III) 氧化
与其他过氧化物相比,过二硫酸盐(PDS)成本相对较低且稳定性较高,是一种颇具吸引力的消除水污染物的氧化剂。它需要活化才能应用,特别优选非自由基生产工艺以获得高选择性并减少普遍存在的氯离子对活性物质的无用消耗。不幸的是,目前大多数 PDS 非自由基氧化过程对惰性污染物无效。在这里,我们报告了一种由 CuO-MgO 复合材料引发的新型非自由基 PDS 氧化过程,可有效去除难熔的碘化造影剂化合物 (ICM)。优化的 CuO-MgO 复合材料在激活 PDS 去除碘帕醇(一种典型的难处理 ICM)方面的效率比 CuO 高 18 倍,并且 Cu2+ 浸出率低得多。有趣的是,增强的氧化与自由基、活性氧 (ROS) 或高价 Cu(III) 无关。我们通过一系列的表面表征和密度泛函理论计算发现,CuO-MgO复合材料具有高电子密度的Cu2+-Mg2+异质结,因此比单独的CuO对于PDS活化具有更高的活性。 PDS以内球模式与复合材料表面相互作用;因此,表面结合的 PDS 直接从惰性污染物中提取电子。由于氧化能力比传统自由基低,ICM 探针的 PDS/CuO-MgO 氧化对氯不敏感,并且产生较少的羟基和硝基降解产物。该研究强调,Cu2+-Mg2+异质结在增强PDS对惰性污染物的非自由基和非ROS氧化能力方面可以发挥重要作用。
更新日期:2024-01-22
中文翻译:

CuO-MgO 复合材料显着增强了惰性污染物的非自由基、非 ROS 和非 Cu(III) 氧化
与其他过氧化物相比,过二硫酸盐(PDS)成本相对较低且稳定性较高,是一种颇具吸引力的消除水污染物的氧化剂。它需要活化才能应用,特别优选非自由基生产工艺以获得高选择性并减少普遍存在的氯离子对活性物质的无用消耗。不幸的是,目前大多数 PDS 非自由基氧化过程对惰性污染物无效。在这里,我们报告了一种由 CuO-MgO 复合材料引发的新型非自由基 PDS 氧化过程,可有效去除难熔的碘化造影剂化合物 (ICM)。优化的 CuO-MgO 复合材料在激活 PDS 去除碘帕醇(一种典型的难处理 ICM)方面的效率比 CuO 高 18 倍,并且 Cu2+ 浸出率低得多。有趣的是,增强的氧化与自由基、活性氧 (ROS) 或高价 Cu(III) 无关。我们通过一系列的表面表征和密度泛函理论计算发现,CuO-MgO复合材料具有高电子密度的Cu2+-Mg2+异质结,因此比单独的CuO对于PDS活化具有更高的活性。 PDS以内球模式与复合材料表面相互作用;因此,表面结合的 PDS 直接从惰性污染物中提取电子。由于氧化能力比传统自由基低,ICM 探针的 PDS/CuO-MgO 氧化对氯不敏感,并且产生较少的羟基和硝基降解产物。该研究强调,Cu2+-Mg2+异质结在增强PDS对惰性污染物的非自由基和非ROS氧化能力方面可以发挥重要作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号