当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Methyl 3-Hydroxyisoxazole-5-Carboxylate Via Bromination of Dimethyl Fumarate under Photoflow Conditions and Its Safe Homologation into 3-(3-Methoxyisoxazol-5-yl) Propanoic Acid
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-01-22 , DOI: 10.1021/acs.oprd.3c00334 Cédric Bürki 1 , Marco Künzli 1 , Patric Dörrwächter 1 , Patrick Wallquist-Franke 1 , Larry Y. Wang 2 , Feng Zhang 2 , Gabriel Schäfer 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-01-22 , DOI: 10.1021/acs.oprd.3c00334 Cédric Bürki 1 , Marco Künzli 1 , Patric Dörrwächter 1 , Patrick Wallquist-Franke 1 , Larry Y. Wang 2 , Feng Zhang 2 , Gabriel Schäfer 1
Affiliation
|
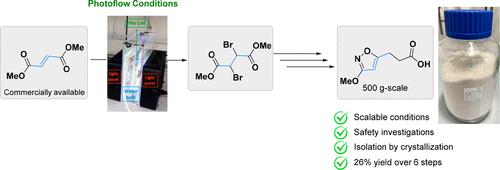
The route scouting and development activities toward a safe and scalable manufacturing route for 3-(3-methoxyisoxazole-5-yl) propanoic acid are outlined in this article. In a first step, methyl 3-hydroxy-5-isoxazolecarboxylate (CAS: 10068-07-2) was prepared on a kilogram scale via bromination of dimethyl fumarate under photoflow conditions followed by condensation with hydroxyurea. This intermediate was then two-carbon homologated by a sequence of ester reduction, chlorination, and nucleophilic substitution with commercially available triethylmethanetricarboxylate. Subsequently, a double decarboxylation event unveiled the desired building block 3-(3-methoxyisoxazole-5-yl) propanoic acid. During our development activities, a preliminary safety assessment of the intermediates by differential scanning calorimetry revealed that a high thermal decomposition energy was recorded for the isoxazole heterocycle. Therefore, our route scouting activities and isolation strategies needed to be carefully assessed under the guidance of the Oxygen balance/Rule of 6/Explosive functional group/Onset temperature/Scale (OREOS) safety assessment. Finally, to highlight our development activities, a kg-scale campaign demonstrated the scalability of the new route.
中文翻译:

光流条件下富马酸二甲酯溴化合成3-羟基异恶唑-5-甲酸甲酯及其安全同系化为3-(3-甲氧基异恶唑-5-基)丙酸
本文概述了 3-(3-甲氧基异恶唑-5-基)丙酸安全且可扩展的生产路线的探索和开发活动。第一步,通过富马酸二甲酯在光流条件下溴化,然后与羟基脲缩合,制备公斤级的 3-羟基-5-异恶唑甲酸甲酯(CAS:10068-07-2)。然后通过一系列酯还原、氯化和用市售的甲烷三甲酸三乙酯进行亲核取代,将该中间体进行二碳同系化。随后,双重脱羧事件揭示了所需的结构单元 3-(3-甲氧基异恶唑-5-基)丙酸。在我们的开发活动中,通过差示扫描量热法对中间体进行的初步安全评估表明,异恶唑杂环记录了高热分解能。因此,我们的路线探索活动和隔离策略需要在氧平衡/6规则/爆炸功能组/起始温度/规模(OREOS)安全评估的指导下仔细评估。最后,为了突出我们的开发活动,公斤级的活动展示了新路线的可扩展性。
更新日期:2024-01-22
中文翻译:

光流条件下富马酸二甲酯溴化合成3-羟基异恶唑-5-甲酸甲酯及其安全同系化为3-(3-甲氧基异恶唑-5-基)丙酸
本文概述了 3-(3-甲氧基异恶唑-5-基)丙酸安全且可扩展的生产路线的探索和开发活动。第一步,通过富马酸二甲酯在光流条件下溴化,然后与羟基脲缩合,制备公斤级的 3-羟基-5-异恶唑甲酸甲酯(CAS:10068-07-2)。然后通过一系列酯还原、氯化和用市售的甲烷三甲酸三乙酯进行亲核取代,将该中间体进行二碳同系化。随后,双重脱羧事件揭示了所需的结构单元 3-(3-甲氧基异恶唑-5-基)丙酸。在我们的开发活动中,通过差示扫描量热法对中间体进行的初步安全评估表明,异恶唑杂环记录了高热分解能。因此,我们的路线探索活动和隔离策略需要在氧平衡/6规则/爆炸功能组/起始温度/规模(OREOS)安全评估的指导下仔细评估。最后,为了突出我们的开发活动,公斤级的活动展示了新路线的可扩展性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号