当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Visible-Light-Induced Synthesis of 1,2-Dicarboxyl Compounds from Carbon Dioxide, Carbamoyl-dihydropyridine, and Styrene
Organic Letters ( IF 4.9 ) Pub Date : 2024-01-22 , DOI: 10.1021/acs.orglett.3c04015 Kimberly Benedetti Vega 1, 2 , André Luiz Carvalho de Oliveira 1 , Burkhard König 2 , Márcio Weber Paixão 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-01-22 , DOI: 10.1021/acs.orglett.3c04015 Kimberly Benedetti Vega 1, 2 , André Luiz Carvalho de Oliveira 1 , Burkhard König 2 , Márcio Weber Paixão 1
Affiliation
|
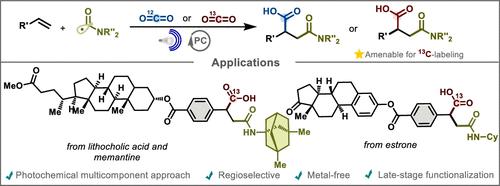
β-Amidated carboxylic acids, or succinamic acid derivatives, constitute a valuable chemical scaffold with broad applications in pharmaceuticals, agrochemicals, and polymer sciences. Herein, we report a redox-neutral multicomponent reaction for the synthesis of succinamic acid derivatives in good yields. This protocol involves styrene, CO2 and 1,4-carbamoyl-dihydropyridine as radical precursors. The method exhibits a broad substrate scope under mild reaction conditions, including late-stage functionalization. Moreover, by employing 13CO2, the method enables the synthesis of labeled 1,2-dicarboxylic compounds.
中文翻译:

二氧化碳、氨基甲酰基二氢吡啶和苯乙烯可见光诱导合成 1,2-二羧基化合物
β-酰胺化羧酸或琥珀酸衍生物构成了一种有价值的化学支架,在药物、农用化学品和高分子科学中具有广泛的应用。在此,我们报道了一种以良好产率合成琥珀酸衍生物的氧化还原中性多组分反应。该方案涉及苯乙烯、CO 2和1,4-氨基甲酰基-二氢吡啶作为自由基前体。该方法在温和的反应条件下表现出广泛的底物范围,包括后期功能化。此外,通过使用13 CO 2,该方法能够合成标记的1,2-二羧酸化合物。
更新日期:2024-01-22
中文翻译:

二氧化碳、氨基甲酰基二氢吡啶和苯乙烯可见光诱导合成 1,2-二羧基化合物
β-酰胺化羧酸或琥珀酸衍生物构成了一种有价值的化学支架,在药物、农用化学品和高分子科学中具有广泛的应用。在此,我们报道了一种以良好产率合成琥珀酸衍生物的氧化还原中性多组分反应。该方案涉及苯乙烯、CO 2和1,4-氨基甲酰基-二氢吡啶作为自由基前体。该方法在温和的反应条件下表现出广泛的底物范围,包括后期功能化。此外,通过使用13 CO 2,该方法能够合成标记的1,2-二羧酸化合物。















































 京公网安备 11010802027423号
京公网安备 11010802027423号