Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-01-18 , DOI: 10.1016/j.jconrel.2024.01.022 Mengyun Peng 1 , Hongyan Dong 1 , Meiyu Shao 1 , Xiaoqing Zhang 1 , Jiamei Sun 1 , Chuan Ding 1 , Xin Han 1 , Qiao Yang 1 , Xianan Sang 1 , Gang Cao 1
|
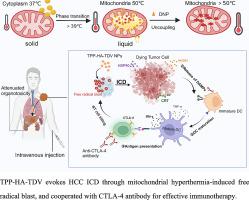
Hepatocellular carcinoma (HCC) is an immunosuppressive tumor associated with high mortality. Photothermal and photodynamic therapies have been applied to induce immunogenic cell death (ICD) in HCC, successfully eliciting immune responses but facing limitations in penetration depth in clinical trials. Here, intrinsic mitochondrial hyperthermia was used to trigger thermosensitive drug release. The mitochondria were further self-heated through 2,4-dinitrophenol uncoupling, dramatically promoting free radical initiation and inducing tumor ICD. The synthesized mitochondrial-targeting TPP-HA-TDV nanoparticles specifically generated free radicals in the mitochondria without external stimulation, and obviously enhanced the release of ICD markers, subsequently evoking immune responses. The results showed that mitochondrial hyperthermia could be an endogenous target for thermosensitive drug release. Furthermore, self-heating mitochondria-induced free radical blast could be an efficient therapeutic for deep-seated tumor therapy.
中文翻译:

自加热线粒体诱导的自由基爆炸用于免疫原性细胞死亡刺激和 HCC 免疫治疗
肝细胞癌(HCC)是一种与高死亡率相关的免疫抑制肿瘤。光热和光动力疗法已应用于诱导肝癌中的免疫原性细胞死亡(ICD),成功引发免疫反应,但在临床试验中面临渗透深度的限制。在这里,内在的线粒体高温被用来触发热敏药物的释放。线粒体通过 2,4-二硝基苯酚解偶联进一步自加热,显着促进自由基引发并诱导肿瘤 ICD。合成的靶向线粒体的TPP-HA-TDV纳米颗粒无需外部刺激即可在线粒体中特异性产生自由基,并明显增强ICD标记物的释放,随后引发免疫反应。结果表明,线粒体高温可能是热敏药物释放的内源性靶标。此外,自加热线粒体诱导的自由基爆炸可能是深层肿瘤治疗的有效疗法。































 京公网安备 11010802027423号
京公网安备 11010802027423号