Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced bulk and interfacial charge transfer in Fe:VOPO4 modified Mo:BiVO4 photoanodes for photoelectrochemical water splitting
eScience ( IF 42.9 ) Pub Date : 2024-01-20 , DOI: 10.1016/j.esci.2024.100242
Bing He , Yu Cao , Kaijie Lin , Mingjie Wu , Yunhai Zhu , Xun Cui , Liang Hu , Yingkui Yang , Xueqin Liu
eScience ( IF 42.9 ) Pub Date : 2024-01-20 , DOI: 10.1016/j.esci.2024.100242
Bing He , Yu Cao , Kaijie Lin , Mingjie Wu , Yunhai Zhu , Xun Cui , Liang Hu , Yingkui Yang , Xueqin Liu
|
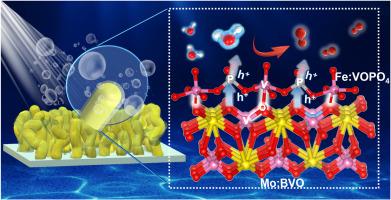
Bismuth vanadate (BiVO4 ) is a promising photoanode material for photoelectrochemical (PEC) water oxidation. However, its performance is greatly hindered by poor bulk and interfacial charge transfer. Herein, to address this issue, iron doped vanadyl phosphate (Fe:VOPO4 ) was grafted on molybdenum doped BiVO4 (Mo:BiVO4 ) for significantly enhancing charge transfer and oxygen evolution kinetics simultaneously. Consequently, the resultant Fe:VOPO4 /Mo:BVO4 photoanode exhibits a remarkable photocurrent density of 6.59 mA cm−2 at 1.23 V versus the reversible hydrogen electrode (VRHE ) under AM 1.5G illumination, over approximately 5.5 times as high as that of pristine BiVO4 . Systematic studies have demonstrated that the hopping activation energy of small polarons is significantly reduced due to the Mo doping, resulting in accelerated bulk charge transfer. More importantly, the deposition of Fe:VOPO4 promotes the interfacial charge transfer between Mo:BiVO4 and Fe:VOPO4 via the construction of V–O–V and P–O bonds, in addition to facilitating water splitting kinetics. This work provides a general strategy for optimizing charge transfer process, especially at the interface between photoanodes and cocatalysts.
中文翻译:

Fe:VOPO4改性Mo:BiVO4光阳极在光电化学分解水中的应用增强本体电荷转移和界面电荷转移
钒酸铋 (BiVO4) 是一种很有前途的用于光电化学 (PEC) 水氧化的光阳极材料。然而,其性能受到体积和界面电荷转移不良的极大阻碍。在此,为了解决这个问题,将铁掺杂的磷酸钒 (Fe:VOPO4) 接枝到掺钼的 BiVO4 (Mo:BiVO4) 上,以同时显着增强电荷转移和析氧动力学。因此,在 AM 1.5G 照明下,与可逆氢电极 (VRHE) 相比,所得的 Fe:VOPO4/Mo:BVO4 光阳极在 1.23 V 下表现出 6.59 mA cm-2 的显着光电流密度,大约是原始 BiVO4 的 5.5 倍。系统研究表明,由于 Mo 掺杂,小极化子的跳跃活化能显着降低,导致本体电荷转移加速。更重要的是,Fe:VOPO4 的沉积除了促进分解水动力学外,还通过构建 V-O-V 和 P-O 键促进了 Mo:BiVO4 和 Fe:VOPO4 之间的界面电荷转移。这项工作为优化电荷转移过程提供了一种通用策略,尤其是在光阳极和助催化剂之间的界面处。
更新日期:2024-01-20
中文翻译:

Fe:VOPO4改性Mo:BiVO4光阳极在光电化学分解水中的应用增强本体电荷转移和界面电荷转移
钒酸铋 (BiVO4) 是一种很有前途的用于光电化学 (PEC) 水氧化的光阳极材料。然而,其性能受到体积和界面电荷转移不良的极大阻碍。在此,为了解决这个问题,将铁掺杂的磷酸钒 (Fe:VOPO4) 接枝到掺钼的 BiVO4 (Mo:BiVO4) 上,以同时显着增强电荷转移和析氧动力学。因此,在 AM 1.5G 照明下,与可逆氢电极 (VRHE) 相比,所得的 Fe:VOPO4/Mo:BVO4 光阳极在 1.23 V 下表现出 6.59 mA cm-2 的显着光电流密度,大约是原始 BiVO4 的 5.5 倍。系统研究表明,由于 Mo 掺杂,小极化子的跳跃活化能显着降低,导致本体电荷转移加速。更重要的是,Fe:VOPO4 的沉积除了促进分解水动力学外,还通过构建 V-O-V 和 P-O 键促进了 Mo:BiVO4 和 Fe:VOPO4 之间的界面电荷转移。这项工作为优化电荷转移过程提供了一种通用策略,尤其是在光阳极和助催化剂之间的界面处。

































 京公网安备 11010802027423号
京公网安备 11010802027423号