当前位置:
X-MOL 学术
›
J. Mol. Liq.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Volumetric, ultrasonic, conductometric and UV–visible analysis of molecular interactions between metformin hydrochloride and dulcitol in aqueous solutions
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2024-01-19 , DOI: 10.1016/j.molliq.2024.124089
Inesh Kumar , Shashi Kant Lomesh , Dharamvir Singh , Parveen Kumar , Palak Ahir , Sunil Kumar
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2024-01-19 , DOI: 10.1016/j.molliq.2024.124089
Inesh Kumar , Shashi Kant Lomesh , Dharamvir Singh , Parveen Kumar , Palak Ahir , Sunil Kumar
|
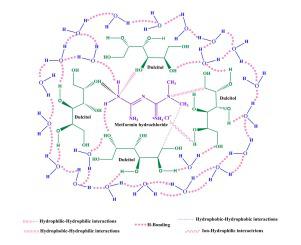
The interactions of a drug with the solvents render imperative information in respect of its behaviour and operation in various areas. The anti-diabetic drug metformin hydrochloride (MF) interacts with water and dulcitol in aqueous solutions, and these interactions have been studied through volumetric, ultrasonic, conductometric, and UV–visible methods The density (), sound velocity (), and molar conductance () of MF in water and aqueous dulcitol (2, 4, and 6 % w/v) have been recorded at four different temperatures to elucidate the interactions of MF with the solvents. The measured parameters were then interpreted in terms of partial molar volume () using Masson’s equation, adiabatic compressibility () using Newton- Laplace equation. In addition to this many other thermodynamic parameters including partial molar volumes of transfer , and Walden’s product were also calculated. These parameters were interpreted to draw insight into MF-MF and MF-solvent interactions. Hepler’s constant and Walden’s temperature coefficient was utilized to determine the structure-making/breaking behaviour of MF in water and aqueous dulcitol solvent systems. In addition to this, spectroscopic analysis using UV–visible spectrophotometer has also been carried out to support the results obtained by various parameters. The rise in intensity and a shift in UV–visible absorption maxima with an increase in MF concentration in the studied solvent systems, suggests that MF interacts strongly with solvents. Moreover, the structure-making nature of MF in the studied solvent system of water and aqueous dulcitol was confirmed by Walden's product and Hepler's constant.
中文翻译:

水溶液中盐酸二甲双胍和卫矛醇之间分子相互作用的体积法、超声波法、电导法和紫外可见分光光度法分析
药物与溶剂的相互作用提供了有关其在各个领域的行为和操作的重要信息。抗糖尿病药物盐酸二甲双胍 (MF) 在水溶液中与水和卫矛醇相互作用,这些相互作用已通过体积法、超声法、电导法和紫外可见光方法进行了研究。密度 ()、声速 () 和摩尔电导在四种不同温度下记录了水和卫矛醇水溶液(2%、4% 和 6% w/v)中 MF 的 (),以阐明 MF 与溶剂的相互作用。然后使用马森方程将测量的参数解释为偏摩尔体积 (),使用牛顿-拉普拉斯方程解释绝热压缩性 ()。除此之外,还计算了许多其他热力学参数,包括转移的部分摩尔体积 和 Walden 乘积。对这些参数进行解释,以深入了解 MF-MF 和 MF-溶剂相互作用。利用赫普勒常数和瓦尔登温度系数来确定 MF 在水和水性卫矛醇溶剂体系中的结构形成/破坏行为。除此之外,还使用紫外可见分光光度计进行了光谱分析,以支持通过各种参数获得的结果。在所研究的溶剂系统中,随着 MF 浓度的增加,强度增加,紫外-可见吸收最大值发生变化,表明 MF 与溶剂有强烈的相互作用。此外,瓦尔登积和赫普勒常数证实了 MF 在所研究的水和含水卫矛醇溶剂体系中的结构形成性质。
更新日期:2024-01-19
中文翻译:

水溶液中盐酸二甲双胍和卫矛醇之间分子相互作用的体积法、超声波法、电导法和紫外可见分光光度法分析
药物与溶剂的相互作用提供了有关其在各个领域的行为和操作的重要信息。抗糖尿病药物盐酸二甲双胍 (MF) 在水溶液中与水和卫矛醇相互作用,这些相互作用已通过体积法、超声法、电导法和紫外可见光方法进行了研究。密度 ()、声速 () 和摩尔电导在四种不同温度下记录了水和卫矛醇水溶液(2%、4% 和 6% w/v)中 MF 的 (),以阐明 MF 与溶剂的相互作用。然后使用马森方程将测量的参数解释为偏摩尔体积 (),使用牛顿-拉普拉斯方程解释绝热压缩性 ()。除此之外,还计算了许多其他热力学参数,包括转移的部分摩尔体积 和 Walden 乘积。对这些参数进行解释,以深入了解 MF-MF 和 MF-溶剂相互作用。利用赫普勒常数和瓦尔登温度系数来确定 MF 在水和水性卫矛醇溶剂体系中的结构形成/破坏行为。除此之外,还使用紫外可见分光光度计进行了光谱分析,以支持通过各种参数获得的结果。在所研究的溶剂系统中,随着 MF 浓度的增加,强度增加,紫外-可见吸收最大值发生变化,表明 MF 与溶剂有强烈的相互作用。此外,瓦尔登积和赫普勒常数证实了 MF 在所研究的水和含水卫矛醇溶剂体系中的结构形成性质。

































 京公网安备 11010802027423号
京公网安备 11010802027423号