当前位置:
X-MOL 学术
›
Sustain. Chem. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultrasensitive spectrofluorimetric approach for quantitation of the novel antiparkinsonian drug safinamide in different matrices at nanogram levels: Assessment of greenness and whiteness profiles
Sustainable Chemistry and Pharmacy ( IF 5.5 ) Pub Date : 2024-01-19 , DOI: 10.1016/j.scp.2024.101448 Galal Magdy , Aya Saad Radwan , Heba Elmansi , Fathalla Belal , Mai Abd El-Aziz , Omar M El-Abassy
Sustainable Chemistry and Pharmacy ( IF 5.5 ) Pub Date : 2024-01-19 , DOI: 10.1016/j.scp.2024.101448 Galal Magdy , Aya Saad Radwan , Heba Elmansi , Fathalla Belal , Mai Abd El-Aziz , Omar M El-Abassy
|
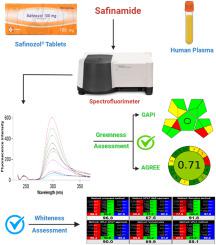
The present work introduces the first spectrofluorimetric approach for the estimation of the novel antiparkinsonian drug safinamide mesylate in pharmaceutical formulations and human plasma. The developed method depends on measuring its native fluorescence in methanol. The measurements were carried out at 299 nm upon excitation at 226 nm. The effects of pH, different solvents, and organized media were thoroughly investigated and optimized. Methanol was found to be the most suitable solvent, and Britton-Robinson buffer (0.02 M) with pH 4 was used. The fluorescence quantum yield of the studied drug was calculated and found to be as high as 44.5 %. The method was validated following the International Council of Harmonization (ICH) validation recommendations. The linearity range was found to be 50.0–2000.0 ng/mL, reaching limits of detection and quantitation of 11.58 and 35.08 ng/mL, respectively. The method's high sensitivity permitted its application to spiked human plasma samples with high percentage recoveries (97.52–101.27%) and low percentage RSD values (<1.24). The designed method was also applied for the estimation of the cited drug in commercial Safinozol® tablets, and the results obtained agreed statistically with those of the comparison method. Furthermore, the greenness and whiteness profiles of the developed method were evaluated for the accomplishment of a complete eco-friendliness profile of the designed methodology.
中文翻译:

超灵敏荧光分光光度法对不同基质中纳克级新型抗帕金森病药物沙芬酰胺进行定量:评估绿度和白度曲线
目前的工作介绍了第一个用于评估药物制剂和人血浆中新型抗帕金森病药物甲磺酸沙芬酰胺的荧光分光光度法。所开发的方法取决于测量其在甲醇中的天然荧光。测量在 226 nm 激发后在 299 nm 处进行。pH值、不同溶剂和有组织的介质的影响被彻底研究和优化。发现甲醇是最合适的溶剂,并使用 pH 4 的 Britton-Robinson 缓冲液 (0.02 M)。计算发现该药物的荧光量子产率高达44.5%。该方法根据国际协调委员会 (ICH) 验证建议进行了验证。线性范围为 50.0–2000.0 ng/mL,检测限和定量限分别为 11.58 和 35.08 ng/mL。该方法的高灵敏度使其能够应用于加标的人血浆样品,并具有高回收率 (97.52–101.27%) 和低百分比 RSD 值 (<1.24)。所设计的方法也应用于市售Safinozol®片剂中所引用药物的评估,所得结果与对比方法的结果在统计学上一致。此外,还评估了所开发方法的绿度和白度特征,以实现所设计方法的完整生态友好特征。
更新日期:2024-01-19
中文翻译:

超灵敏荧光分光光度法对不同基质中纳克级新型抗帕金森病药物沙芬酰胺进行定量:评估绿度和白度曲线
目前的工作介绍了第一个用于评估药物制剂和人血浆中新型抗帕金森病药物甲磺酸沙芬酰胺的荧光分光光度法。所开发的方法取决于测量其在甲醇中的天然荧光。测量在 226 nm 激发后在 299 nm 处进行。pH值、不同溶剂和有组织的介质的影响被彻底研究和优化。发现甲醇是最合适的溶剂,并使用 pH 4 的 Britton-Robinson 缓冲液 (0.02 M)。计算发现该药物的荧光量子产率高达44.5%。该方法根据国际协调委员会 (ICH) 验证建议进行了验证。线性范围为 50.0–2000.0 ng/mL,检测限和定量限分别为 11.58 和 35.08 ng/mL。该方法的高灵敏度使其能够应用于加标的人血浆样品,并具有高回收率 (97.52–101.27%) 和低百分比 RSD 值 (<1.24)。所设计的方法也应用于市售Safinozol®片剂中所引用药物的评估,所得结果与对比方法的结果在统计学上一致。此外,还评估了所开发方法的绿度和白度特征,以实现所设计方法的完整生态友好特征。































 京公网安备 11010802027423号
京公网安备 11010802027423号