Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2024-01-20 , DOI: 10.1016/j.bmc.2024.117608
Yunsheng Xu 1 , Wei Zhao 1 , Xinyi Zhang 1 , Xihua Yu 1 , Yinbo Chen 1 , Zhenghai Wang 1 , Yong Chu 2 , Xueyan Zhu 1 , Peng Zhang 1
|
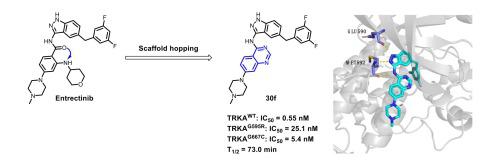
Tropomyosin receptor kinases (TRKs), the superfamily of transmembrane receptor tyrosine kinases, have recently become an attractive method for precision anticancer therapies since the approval of Larotrectinib and Entrectinib by FDA. Herein, we reported the discovery of a series of novel indazolylaminoquinazoline and indazolylaminoindazole as TRK inhibitors. The representative compound 30f exhibited good inhibitory activity against TRKWT, TRKG595R and TRKG667C with IC50 values of 0.55 nM, 25.1 nM and 5.4 nM, respectively. The compound also demonstrated potent superior to Larotrectinib antiproliferative activity against a panel of Ba/F3 cell lines transformed with both NTRK wild type and mutant fusions (IC50 = 10–200 nM). In addition, compound 30f exhibited good in vitro metabolic stability (T1/2 = 73.0 min), indicating that the quinazoline derivatives may have better metabolic stability. Finally, the binding mode of compound 30f predicted by molecular docking well explained the good enzyme inhibitory activity of indazolylaminoquinazoline compounds as TRK inhibitor. Thus, compound 30f can be used as a promising lead molecule for further structural optimization.
中文翻译:

作为有效原肌球蛋白受体激酶 (TRK) 抑制剂的吲唑氨基喹唑啉衍生物的设计、合成和评估
原肌球蛋白受体激酶(TRK)是跨膜受体酪氨酸激酶的超家族,自FDA批准Larotrectinib 和 Entrectinib 以来,最近已成为精准抗癌治疗的一种有吸引力的方法。在此,我们报道了一系列新型吲唑氨基喹唑啉和吲唑氨基吲唑作为 TRK 抑制剂的发现。代表性化合物30f对TRK WT 、TRK G595R和TRK G667C表现出良好的抑制活性,IC 50值分别为0.55 nM、25.1 nM和5.4 nM。该化合物还对一组用 NTRK 野生型和突变融合体转化的 Ba/F3 细胞系表现出优于 Larotrectinib 的抗增殖活性(IC 50 = 10–200 nM)。此外,化合物30f表现出良好的体外代谢稳定性(T 1/2 = 73.0 min),表明喹唑啉衍生物可能具有更好的代谢稳定性。最后,通过分子对接预测的化合物30f的结合模式很好地解释了吲唑氨基喹唑啉类化合物作为TRK抑制剂具有良好的酶抑制活性。因此,化合物30f可以用作进一步结构优化的有前景的先导分子。

































 京公网安备 11010802027423号
京公网安备 11010802027423号