Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-01-18 , DOI: 10.1016/j.jcat.2024.115303 Zhen-Yu Zhang , Ting Li , Xia-Li Sun , De-Cun Luo , Ji-Long Yao , Gui-Dong Yang , Tao Xie
|
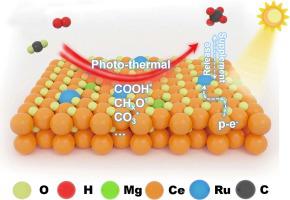
It is a promising green technology to reduce CO2 to CH4 by using renewable solar energy driving photo-thermal catalysis route. However, the efficiency of photo-thermal catalytic CO2 methanation remained to be improved, and the mechanism of this reaction still required further investigation. In this paper, a Ru/Mg-CeO2 catalyst was designed based on the concepts of single atom catalyst and photo-thermal catalysis concept. The catalyst achieved the CH4 formation rate of 469 mmol·gcat−1·h−1 at 400 °C under photo-thermal conditions with good catalytic stability. Besides, the superiority of the single-atom of Ru, Ru-O-Ce structure and doping of alkaline metals in catalytic process was proved by a series of physicochemical and optical characterization, and electron migration direction from Ce and Mg elements to Ru sites through Ru-O-Ce and Ru-O-Mg path under photo-thermal catalysis was determined by ISI-XPS experiment. Finally, the comprehensive reaction mechanism and reversible dynamic structural evolution process between Ru-O-Ce and Ru-Ov-Ce structures were investigated by in situ DRIFTs experiments, and the source of the excellent performance and structure-function relationship of the catalyst was explored, which provided the theoretical and practical basis for the development of catalyst and industrial application of the reaction.
中文翻译:

Ru/Mg-CeO2 单原子催化剂的高效光热催化 CO2 甲烷化和动态结构演化
利用可再生太阳能驱动光热催化路线将CO 2 还原为CH 4 是一项很有前景的绿色技术。然而,光热催化CO2甲烷化的效率仍有待提高,该反应的机理仍需进一步研究。本文基于单原子催化剂和光热催化概念设计了Ru/Mg-CeO 2 催化剂。该催化剂在400 ℃光热条件下CH 4 生成率为469 mmol·gcat −1 ·h −1 ,具有良好的催化稳定性。此外,通过一系列物理化学和光学表征以及从Ce和Mg元素到Ru位点的电子迁移方向,证明了Ru单原子、Ru-O-Ce结构和碱金属掺杂在催化过程中的优越性。通过ISI-XPS实验确定了光热催化下Ru-O-Ce和Ru-O-Mg的路径。最后,通过原位DRIFTs实验研究了Ru-O-Ce和Ru-O v -Ce结构之间的综合反应机理和可逆动态结构演化过程,以及其优异性能和结构的来源——探讨了催化剂的作用关系,为催化剂的开发和反应的工业化应用提供了理论和实践基础。





























 京公网安备 11010802027423号
京公网安备 11010802027423号