当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Intra- and Intermolecular Haloetherification of Enones: An Efficient Approach to (−)-Centrolobine
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-10-17 00:00:00 , DOI: 10.1021/acscatal.6b02048 Pengfei Zhou 1 , Yunfei Cai 1 , Xia Zhong 1 , Weiwei Luo 1 , Tengfei Kang 1 , Jun Li 1 , Xiaohua Liu 1 , Lili Lin 1 , Xiaoming Feng 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-10-17 00:00:00 , DOI: 10.1021/acscatal.6b02048 Pengfei Zhou 1 , Yunfei Cai 1 , Xia Zhong 1 , Weiwei Luo 1 , Tengfei Kang 1 , Jun Li 1 , Xiaohua Liu 1 , Lili Lin 1 , Xiaoming Feng 1
Affiliation
|
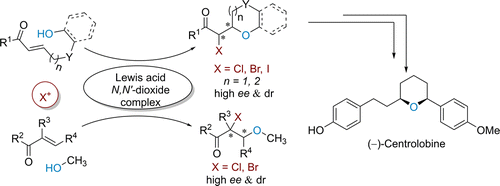
A catalytic asymmetric intra- and intermolecular haloetherification of electron-deficient alkenes (halogen = Cl, Br, I) has been realized by the use of chiral metal complexes of N,N′-dioxides. In the presence of a chiral Fe(III) complex, a series of tetrahydropyran derivatives were obtained in good yields (up to 99% yield) with a high level of enantioselectivities (up to 97% ee). Promoted by a chiral Ce(III) complex, chiral oxepane derivatives could be given in good results. Moreover, the intermolecular haloetherification of chalcones catalyzed by Sc(III) complex using MeOH as nucleophile is demonstrated. This methodology also can be successfully applied to the synthesis of (−)-Centrolobine. Meanwhile, a reasonable reaction mechanism was proposed.
中文翻译:

烯类的催化不对称分子内和分子间卤代醚化:(-)-Centrolobine的一种有效方法
缺电子烯烃(卤素= Cl,Br,I)的催化不对称分子内和分子间卤代醚化反应已通过使用N,N'-二氧化物的手性金属配合物实现。在手性Fe(III)络合物的存在下,以良好的收率(最高99%的收率)和高的对映选择性(最高97%ee)获得了一系列四氢吡喃衍生物。在手性Ce(III)配合物的促进下,手性氧杂环丁烷衍生物可以得到很好的结果。而且,证明了使用MeOH作为亲核试剂,由Sc(III)配合物催化的查耳酮的分子间卤醚化。该方法也可以成功地应用于(-)-Centrolobine的合成。同时,提出了合理的反应机理。
更新日期:2016-10-17
中文翻译:

烯类的催化不对称分子内和分子间卤代醚化:(-)-Centrolobine的一种有效方法
缺电子烯烃(卤素= Cl,Br,I)的催化不对称分子内和分子间卤代醚化反应已通过使用N,N'-二氧化物的手性金属配合物实现。在手性Fe(III)络合物的存在下,以良好的收率(最高99%的收率)和高的对映选择性(最高97%ee)获得了一系列四氢吡喃衍生物。在手性Ce(III)配合物的促进下,手性氧杂环丁烷衍生物可以得到很好的结果。而且,证明了使用MeOH作为亲核试剂,由Sc(III)配合物催化的查耳酮的分子间卤醚化。该方法也可以成功地应用于(-)-Centrolobine的合成。同时,提出了合理的反应机理。































 京公网安备 11010802027423号
京公网安备 11010802027423号