当前位置:
X-MOL 学术
›
J. Mater. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The impact of vacancy defective MgH2 (001)/(110) surface on the dehydrogenation of MgH2@Ni-CNTs: A mechanistic investigation
Journal of Materials Science & Technology ( IF 11.2 ) Pub Date : 2024-01-19 , DOI: 10.1016/j.jmst.2023.11.072
Congwen Duan , Xinya Wang , Haimei Wang , Mengmeng Wu , Yuchen Fan , Jinhui Wu , Ting Qu , Bogu Liu , Lianxi Hu , Poqian Liang , Fei Wang , Ying Wu
Journal of Materials Science & Technology ( IF 11.2 ) Pub Date : 2024-01-19 , DOI: 10.1016/j.jmst.2023.11.072
Congwen Duan , Xinya Wang , Haimei Wang , Mengmeng Wu , Yuchen Fan , Jinhui Wu , Ting Qu , Bogu Liu , Lianxi Hu , Poqian Liang , Fei Wang , Ying Wu
|
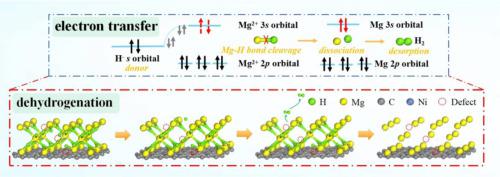
The vacancy defect exhibits a remarkable improvement in the dehydriding property of MgH@Ni-CNTs. However, the corresponding mechanism is still not fully understood. Herein, the impact of vacancy defects on the dehydrogenation properties of MgH@Ni-CNTs was studied by DFT simulation, and the corresponding models were constructed based on MS. The dehydrogenation process of MgH can be regarded as the dissociation of Mg-H and desorption of H from the MgH surface. In view of the whole dehydrogenation, the dissociation of H is the rate-determining step, which is the main reason for restricting the dehydrogenation kinetics. Compared with vacancy vacancy-defective MgH (001) surface, the appearance of vacancy defects on the (110) surface substantially reduces the energy barrier required for H dissociation to 0.070 Ha. The reason is that vacancy defects accelerate the transition of electrons from the H s orbit to the Mg 3s orbit, resulting in a decrement of the Mg-H bond strength, which makes H atoms more easily dissociated from the MgH (110) surface. Therefore, the existence of vacancy defects improves the dehydriding kinetic of MgH. Most importantly, this research offers crucial directions for developing hydrogen storage materials as well as a potential fix for the slow dehydrogenation kinetics of nano-confined MgH.
中文翻译:

空位缺陷 MgH2 (001)/(110) 表面对 MgH2@Ni-CNTs 脱氢的影响:机理研究
空位缺陷显着改善了MgH@Ni-CNTs的脱氢性能。然而,相应的机制仍不完全清楚。本文通过DFT模拟研究了空位缺陷对MgH@Ni-CNTs脱氢性能的影响,并基于MS构建了相应的模型。 MgH的脱氢过程可以看作是Mg-H的解离和H从MgH表面的解吸。从整个脱氢反应来看,H的解离是限速步骤,是限制脱氢动力学的主要原因。与空位缺陷的MgH(001)表面相比,(110)表面空位缺陷的出现将H离解所需的能垒大幅降低至0.070 Ha。原因是空位缺陷加速了电子从H s 轨道向Mg 3s 轨道的跃迁,导致Mg-H键强度降低,使得H原子更容易从MgH(110)表面解离。因此,空位缺陷的存在改善了MgH的脱氢动力学。最重要的是,这项研究为开发储氢材料提供了关键方向,并为纳米限制的 MgH 缓慢脱氢动力学提供了潜在的解决方案。
更新日期:2024-01-19
中文翻译:

空位缺陷 MgH2 (001)/(110) 表面对 MgH2@Ni-CNTs 脱氢的影响:机理研究
空位缺陷显着改善了MgH@Ni-CNTs的脱氢性能。然而,相应的机制仍不完全清楚。本文通过DFT模拟研究了空位缺陷对MgH@Ni-CNTs脱氢性能的影响,并基于MS构建了相应的模型。 MgH的脱氢过程可以看作是Mg-H的解离和H从MgH表面的解吸。从整个脱氢反应来看,H的解离是限速步骤,是限制脱氢动力学的主要原因。与空位缺陷的MgH(001)表面相比,(110)表面空位缺陷的出现将H离解所需的能垒大幅降低至0.070 Ha。原因是空位缺陷加速了电子从H s 轨道向Mg 3s 轨道的跃迁,导致Mg-H键强度降低,使得H原子更容易从MgH(110)表面解离。因此,空位缺陷的存在改善了MgH的脱氢动力学。最重要的是,这项研究为开发储氢材料提供了关键方向,并为纳米限制的 MgH 缓慢脱氢动力学提供了潜在的解决方案。

































 京公网安备 11010802027423号
京公网安备 11010802027423号