当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient photocatalytic H2O2 production over K+-intercalated crystalline g-C3N4 with regulated oxygen reduction pathway
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-01-18 , DOI: 10.1016/j.cej.2024.148844
Qiqi Zhang , Bing Wang , Hui Miao , Jun Fan , Tao Sun , Enzhou Liu
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-01-18 , DOI: 10.1016/j.cej.2024.148844
Qiqi Zhang , Bing Wang , Hui Miao , Jun Fan , Tao Sun , Enzhou Liu
|
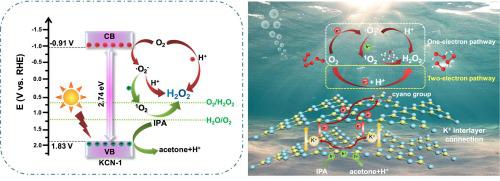
Photocatalytic H2 O2 production via the oxygen reduction pathway is an ideal sustainable technique, while it faces challenges such as sluggish exciton dissociation and charge transfer, as well as limited oxygen adsorption. Here, we successfully synthesized K+ -intercalated porous crystalline carbon nitride nanosheets with cyano group modification (KCN) using a one-step potassium salt-assisted thermal copolymerization strategy. The incorporation of K+ , high crystallinity and cyano group result in an enhancement of light absorption, as well as the promotion of carrier separation and bulk charge migration. The facilitated adsorption and transfer of O2 and H+ accelerate surface reaction kinetics. The optimal H2 O2 production rate of KCN-1 (11.2 mmol‧g−1 ‧h−1 ) is approximately 17 times higher than that of pristine CN (0.616 mmol‧g−1 ‧h−1 ). Additionally, KCN-1 demonstrates exceptional stability and high two-electron reduction selectivity. DFT calculations were employed to elucidate the role of K+ in both structural composition and activity improvement. KCN-1 not only can generate more 2 − for H2 O2 synthesis, but also facilitates the one-step two-electron direct reduction pathway from O2 and a novel formation reaction from 1 O2 intermediate to H2 O2 . This study confirms that effective regulation of molecular structure and optimization of reaction pathways are advantageous for constructing efficient H2 O2 synthesis systems.
中文翻译:

通过 K+ 插层晶体 g-C3N4 与受调节的氧还原途径高效光催化生产 H2O2
通过氧还原途径光催化生产H2O2是一种理想的可持续技术,但它面临着激子解离和电荷转移缓慢以及氧吸附有限等挑战。在这里,我们采用一步钾盐辅助热共聚策略成功合成了具有氰基修饰(KCN)的K+插层多孔结晶氮化碳纳米片。 K+、高结晶度和氰基的引入增强了光吸收,并促进了载流子分离和体电荷迁移。促进 O2 和 H+ 的吸附和转移加速表面反应动力学。 KCN-1的最佳H2O2生成速率(11.2 mmol‧g−1‧h−1)比原始CN(0.616 mmol‧g−1‧h−1)高约17倍。此外,KCN-1 表现出卓越的稳定性和高二电子还原选择性。采用 DFT 计算来阐明 K+ 在结构组成和活性改善方面的作用。 KCN-1不仅可以产生更多的O2−用于H2O2合成,而且还促进了O2的一步双电子直接还原途径以及从1O2中间体到H2O2的新型形成反应。这项研究证实,分子结构的有效调控和反应途径的优化有利于构建高效的H2O2合成系统。
更新日期:2024-01-18
中文翻译:

通过 K+ 插层晶体 g-C3N4 与受调节的氧还原途径高效光催化生产 H2O2
通过氧还原途径光催化生产H2O2是一种理想的可持续技术,但它面临着激子解离和电荷转移缓慢以及氧吸附有限等挑战。在这里,我们采用一步钾盐辅助热共聚策略成功合成了具有氰基修饰(KCN)的K+插层多孔结晶氮化碳纳米片。 K+、高结晶度和氰基的引入增强了光吸收,并促进了载流子分离和体电荷迁移。促进 O2 和 H+ 的吸附和转移加速表面反应动力学。 KCN-1的最佳H2O2生成速率(11.2 mmol‧g−1‧h−1)比原始CN(0.616 mmol‧g−1‧h−1)高约17倍。此外,KCN-1 表现出卓越的稳定性和高二电子还原选择性。采用 DFT 计算来阐明 K+ 在结构组成和活性改善方面的作用。 KCN-1不仅可以产生更多的O2−用于H2O2合成,而且还促进了O2的一步双电子直接还原途径以及从1O2中间体到H2O2的新型形成反应。这项研究证实,分子结构的有效调控和反应途径的优化有利于构建高效的H2O2合成系统。







































 京公网安备 11010802027423号
京公网安备 11010802027423号