Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
tert-Butoxide-Mediated Protodeformylative Decarbonylation of α-Quaternary Homobenzaldehydes
SynOpen ( IF 2.0 ) Pub Date : 2024-01-19 , DOI: 10.1055/a-2231-3108 Benjamin J. Stokes 1 , Xiao Cai 2 , Ritter V. Amsbaugh 1 , Lauren J. Drake 1 , Ravi M. A. Kotamraju 1 , Nicholas Javier C. Licauco 1
中文翻译:

叔丁醇介导的 α-季均苯甲醛的原脱甲酰化脱羰
更新日期:2024-01-20
SynOpen ( IF 2.0 ) Pub Date : 2024-01-19 , DOI: 10.1055/a-2231-3108 Benjamin J. Stokes 1 , Xiao Cai 2 , Ritter V. Amsbaugh 1 , Lauren J. Drake 1 , Ravi M. A. Kotamraju 1 , Nicholas Javier C. Licauco 1
Affiliation
|
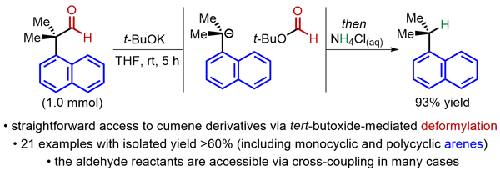
tert-Butoxide mediates the Haller–Bauer-type (protodeformylative) decarbonylation of readily accessed α-quaternary homobenzaldehydes and related compounds at room temperature, generating cumene products. Both geminal dialkyl and geminal diaryl substituents are tolerated. gem-Dimethyls are sufficient for decarbonylation of polycyclic arenyl substrates whereas monocyclic aromatic homobenzaldehydes require cyclic gem-dialkyls or gem-diaryls for significant decarbonylation.
中文翻译:

叔丁醇介导的 α-季均苯甲醛的原脱甲酰化脱羰
叔丁醇在室温下介导容易获得的 α-季均苯甲醛和相关化合物的 Haller-Bauer 型(原脱甲酰化)脱羰,生成异丙苯产物。偕二烷基和偕二芳基取代基都是可以接受的。偕二甲基足以使多环芳基底物脱羰,而单环芳香族同苯甲醛则需要环状偕二烷基或偕二芳基才能显着脱羰。































 京公网安备 11010802027423号
京公网安备 11010802027423号