Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of New 5- or 7-Substituted 3-Nitroimidazo[1,2-a]pyridine Derivatives Using SNAr and Palladium-Catalyzed Reactions To Explore Antiparasitic Structure–Activity Relationships
Synthesis ( IF 2.2 ) Pub Date : 2024-01-19 , DOI: 10.1055/a-2232-8113 Nicolas Primas 1, 2 , Patrice Vanelle 1, 2 , Romain Paoli-Lombardo 1 , Sandra Bourgeade-Delmas 3 , Alix Sournia-Saquet 4 , Caroline Castera-Ducros 1, 2 , Inès Jacquet 1 , Pierre Verhaeghe 4, 5 , Pascal Rathelot 1, 2
中文翻译:

使用 SNAr 和钯催化反应合成新的 5-或 7-取代 3-硝基咪唑并[1,2-a]吡啶衍生物以探索抗寄生虫结构-活性关系
更新日期:2024-01-20
Synthesis ( IF 2.2 ) Pub Date : 2024-01-19 , DOI: 10.1055/a-2232-8113 Nicolas Primas 1, 2 , Patrice Vanelle 1, 2 , Romain Paoli-Lombardo 1 , Sandra Bourgeade-Delmas 3 , Alix Sournia-Saquet 4 , Caroline Castera-Ducros 1, 2 , Inès Jacquet 1 , Pierre Verhaeghe 4, 5 , Pascal Rathelot 1, 2
Affiliation
|
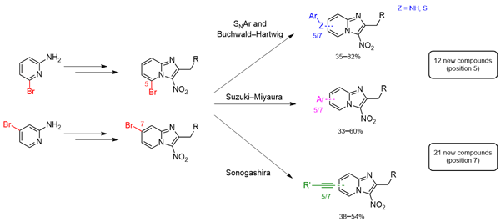
To study the antikinetoplastid structure–activity relationships in a 3-nitroimidazo[1,2-a]pyridine series, we explored the substitution of positions 5 and 7 of the scaffold, developing nucleophilic aromatic substitution reactions and palladium-catalyzed Suzuki–Miyaura, Sonogashira, and Buchwald–Hartwig cross-coupling reactions that had never been reported at these positions in this series. In four steps from 2-amino(bromo)pyridines, 33 original compounds were obtained, allowing a better definition of the antiparasitic pharmacophore.
中文翻译:

使用 SNAr 和钯催化反应合成新的 5-或 7-取代 3-硝基咪唑并[1,2-a]吡啶衍生物以探索抗寄生虫结构-活性关系
为了研究 3-硝基咪唑并[1,2- a ]吡啶系列中的抗动质体结构-活性关系,我们探索了支架 5 和 7 位的取代,开发了亲核芳香取代反应和钯催化的 Suzuki-Miyaura, Sonogashira ,以及本系列中这些位置从未报道过的 Buchwald-Hartwig 交叉偶联反应。通过 2-氨基(溴)吡啶的四个步骤,获得了 33 种原始化合物,从而可以更好地定义抗寄生虫药效团。








































 京公网安备 11010802027423号
京公网安备 11010802027423号