当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unveiling the Bonding Scenario in Metal–Aryne Complexes with EDA-NOCV Analyses
Organometallics ( IF 2.5 ) Pub Date : 2024-01-18 , DOI: 10.1021/acs.organomet.3c00406
Sonam Suthar 1 , Kartik Chandra Mondal 1
Organometallics ( IF 2.5 ) Pub Date : 2024-01-18 , DOI: 10.1021/acs.organomet.3c00406
Sonam Suthar 1 , Kartik Chandra Mondal 1
Affiliation
|
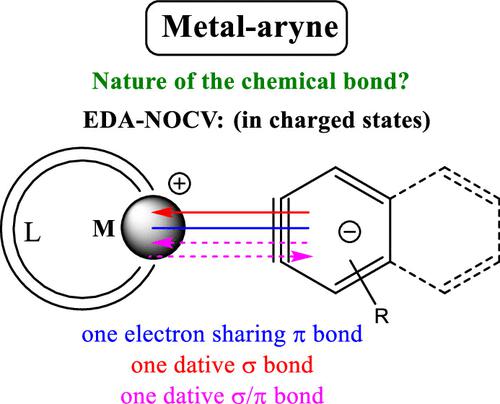
Transition metal-based organometallic compounds have been explained by the Dewar–Chatt–Duncanson (DCD) model, established in 1953, which provides a conceptual framework elucidating the interaction between transition metals and ligands. This interaction involves σ-donation from the ligand to the symmetric vacant d-orbital of the transition metal (TM⃖L), coupled with π-backdonation from a distinctly occupied d-orbital of the transition metal to the suitable empty orbital (mostly antibonding type) on the ligand (TM → L), which leads to the variations in bond lengths in the bonded ligand (typically bond elongation) and vibrational frequencies within ligand bonds (such as C=O, N=N, and C=C of olefins), serving as an indicator of the ligand’s π-accepting strength. One such effective and highly reactive ligand is benzyne/aryne, which is generated in situ and has been stabilized by coordinating to a transition metal. The transition metal–aryne complexes are primarily formed with low-valent early transition metals and late (d10) transition metals. The findings, on employing the EDA-NOCV calculations of different classical textbook examples of experimentally synthesized mononuclear TM–aryne complexes, specifically TM–benzyne complexes, reveal intriguing deviations from the original DCD model and suggest that the bonding interaction of these well-known organometallic complexes occurs between TM and aryne fragments in their ‘electronically charged doublet states’ (as TM+ and aryne–). Notably, when the TM resides within groups IV–IX of the periodic table, the interaction exhibits one dative σ-bond, one electron-sharing π-bond, and one (a few have two) additional dative σ/π bond (D + E). Even though late TM (d10, Ni/Pd/Pt) exhibits the potential to form both dative bonds (D) (in accordance with the DCD model) and D + E interaction between electronically charged fragments, it still slightly favors the later bonding scenario. The major contribution in the bond formation of TM–aryne complexes is from electrostatic interaction energy (ΔEelstat) and the major contribution toward the orbital interaction (ΔEorb) is dominated by the electron sharing π-bond formation.
中文翻译:

通过 EDA-NOCV 分析揭示金属-芳炔配合物中的键合场景
过渡金属基有机金属化合物已通过 1953 年建立的杜瓦-查特-邓肯森 (DCD) 模型进行了解释,该模型提供了阐明过渡金属与配体之间相互作用的概念框架。这种相互作用涉及从配体到过渡金属的对称空 d 轨道 (TM⃖L) 的 σ 供体,以及从过渡金属的明显占据的 d 轨道到合适的空轨道(主要是反键类型)的 π 反供体配体上的振动 (TM → L),导致键合配体中键长的变化(通常是键伸长)和配体键内振动频率的变化(例如烯烃的 C=O、N=N 和 C=C) ,作为配体 π 接受强度的指标。一种有效且高反应性的配体是苯炔/芳炔,它是原位生成的,并通过与过渡金属配位而稳定。过渡金属-芳炔配合物主要由低价的早期过渡金属和晚期(d 10)过渡金属形成。研究结果采用 EDA-NOCV 对实验合成的单核 TM-芳炔配合物(特别是 TM-苯炔配合物)的不同经典教科书示例进行计算,揭示了与原始 DCD 模型的有趣偏差,并表明这些众所周知的有机金属的键合相互作用TM 和芳炔片段之间以“带电子的双峰态”(如 TM +和芳炔–)形成复合物。值得注意的是,当 TM 位于元素周期表的 IV-IX 族时,相互作用表现出一个配位 σ 键、一个共享电子 π 键和一个(少数有两个)附加配位 σ/π 键 (D + E)。尽管后期TM(d 10,Ni/Pd/Pt)表现出在带电片段之间形成配位键(D)(根据DCD模型)和D + E相互作用的潜力,但它仍然稍微有利于后期成键设想。TM-芳炔配合物键形成的主要贡献来自静电相互作用能(Δ E elstat),而轨道相互作用(Δ E orb)的主要贡献则由电子共享π键形成主导。
更新日期:2024-01-18
中文翻译:

通过 EDA-NOCV 分析揭示金属-芳炔配合物中的键合场景
过渡金属基有机金属化合物已通过 1953 年建立的杜瓦-查特-邓肯森 (DCD) 模型进行了解释,该模型提供了阐明过渡金属与配体之间相互作用的概念框架。这种相互作用涉及从配体到过渡金属的对称空 d 轨道 (TM⃖L) 的 σ 供体,以及从过渡金属的明显占据的 d 轨道到合适的空轨道(主要是反键类型)的 π 反供体配体上的振动 (TM → L),导致键合配体中键长的变化(通常是键伸长)和配体键内振动频率的变化(例如烯烃的 C=O、N=N 和 C=C) ,作为配体 π 接受强度的指标。一种有效且高反应性的配体是苯炔/芳炔,它是原位生成的,并通过与过渡金属配位而稳定。过渡金属-芳炔配合物主要由低价的早期过渡金属和晚期(d 10)过渡金属形成。研究结果采用 EDA-NOCV 对实验合成的单核 TM-芳炔配合物(特别是 TM-苯炔配合物)的不同经典教科书示例进行计算,揭示了与原始 DCD 模型的有趣偏差,并表明这些众所周知的有机金属的键合相互作用TM 和芳炔片段之间以“带电子的双峰态”(如 TM +和芳炔–)形成复合物。值得注意的是,当 TM 位于元素周期表的 IV-IX 族时,相互作用表现出一个配位 σ 键、一个共享电子 π 键和一个(少数有两个)附加配位 σ/π 键 (D + E)。尽管后期TM(d 10,Ni/Pd/Pt)表现出在带电片段之间形成配位键(D)(根据DCD模型)和D + E相互作用的潜力,但它仍然稍微有利于后期成键设想。TM-芳炔配合物键形成的主要贡献来自静电相互作用能(Δ E elstat),而轨道相互作用(Δ E orb)的主要贡献则由电子共享π键形成主导。

































 京公网安备 11010802027423号
京公网安备 11010802027423号