当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unveiling the influence of furan and thiophene on the corrosion inhibition capabilities of novel hydrazones derivatives in carbon steel/HCl interface: A dual experimental-theoretical study
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2024-01-19 , DOI: 10.1016/j.colsurfa.2024.133272 Manal Naciri , Siham Skal , Yasmina El Aoufir , Mustapha R. Al-hadeethi , Hassane Lgaz , Hanane Bidi , Mouloud El Moudane , Ahmed Ghanimi , Abdelkebir Bellaouchou
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2024-01-19 , DOI: 10.1016/j.colsurfa.2024.133272 Manal Naciri , Siham Skal , Yasmina El Aoufir , Mustapha R. Al-hadeethi , Hassane Lgaz , Hanane Bidi , Mouloud El Moudane , Ahmed Ghanimi , Abdelkebir Bellaouchou
|
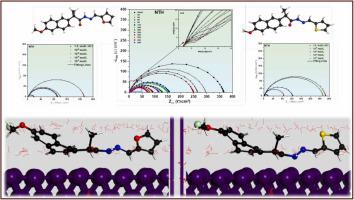
The use of corrosion inhibitors is a critical measure in the preservation of metals. In this research, the performance of two hydrazone derivatives, N′-[()-furan-2-ylmethylidene]− 2-(6-methoxynaphthalen-2-yl)propanehydrazide (NFH) and 2-(6-methoxynaphthalen-2-yl)-N′-[()-thiophen-2-ylmethylidene]propanehydrazide (NTH), was evaluated for their ability to inhibit corrosion of carbon steel in a 1.0 mol/L HCl solution. Electrochemical Impedance Spectroscopy (EIS), Potentiodynamic Polarization (PDP), and Scanning Electron Microscopy with Energy-Dispersive X-ray Spectroscopy (SEM-EDX) were employed to assess the compounds' inhibition performance. The experimental results demonstrated that the efficiency of the inhibitors improved with increasing concentration. NFH reached a 90% threshold, while NTH peaked at 93%. This superior performance of NTH was attributed to its thiophene group, in contrast to the furan moiety in NFH. PDP data revealed that both compounds acted as mixed inhibitors, inhibiting both anodic and cathodic reactions. The EIS results indicated a significant increase in polarization resistance, accompanied by a reduction in the values of effective double-layer capacitance, due to the adsorption of inhibitor molecules. The adsorption of inhibitor molecules conformed to the Langmuir isotherm model, suggesting a combined physicochemical mode of interactions with the carbon steel, which was corroborated by potential of zero charge (PZC) analysis. SEM-EDX analysis confirmed the thiazole derivatives' capacity to protect the metal against corrosion. Quantum Chemical Calculations (QCCs) along with Molecular Dynamics (MD) simulations elucidated the reactivity descriptors and the optimal adsorption configurations on the Fe(110) surface. This exploration underscores the significance of the thiophene moiety in enhancing the adsorptive and corrosion inhibitory properties of the hydrazones, thus providing strategic insights for their future refinement and application.
中文翻译:

揭示呋喃和噻吩对新型腙衍生物在碳钢/HCl界面缓蚀能力的影响:双重实验理论研究
使用腐蚀抑制剂是保护金属的关键措施。在本研究中,两种腙衍生物 N'-[()-呋喃-2-基亚甲基]− 2-(6-甲氧基萘-2-基)丙酰肼 (NFH) 和 2-(6-甲氧基萘-2-) 的性能评估了基)-N'-[()-噻吩-2-基亚甲基]丙酰肼(NTH)在1.0 mol/L HCl溶液中抑制碳钢腐蚀的能力。采用电化学阻抗谱 (EIS)、动电位极化 (PDP) 和扫描电子显微镜与能量色散 X 射线光谱 (SEM-EDX) 来评估化合物的抑制性能。实验结果表明,抑制剂的效率随着浓度的增加而提高。 NFH 达到 90% 的阈值,而 NTH 则达到 93% 的峰值。与 NFH 中的呋喃部分相比,NTH 的这种优异性能归因于其噻吩基团。 PDP 数据显示,这两种化合物充当混合抑制剂,抑制阳极和阴极反应。 EIS 结果表明,由于抑制剂分子的吸附,极化电阻显着增加,同时有效双层电容值降低。抑制剂分子的吸附符合朗缪尔等温线模型,表明与碳钢相互作用的组合物理化学模式,这得到了零电荷电位(PZC)分析的证实。 SEM-EDX 分析证实了噻唑衍生物具有保护金属免受腐蚀的能力。量子化学计算 (QCC) 和分子动力学 (MD) 模拟阐明了反应性描述符和 Fe(110) 表面上的最佳吸附构型。这一探索强调了噻吩部分在增强腙的吸附和腐蚀抑制性能方面的重要性,从而为其未来的精炼和应用提供了战略见解。
更新日期:2024-01-19
中文翻译:

揭示呋喃和噻吩对新型腙衍生物在碳钢/HCl界面缓蚀能力的影响:双重实验理论研究
使用腐蚀抑制剂是保护金属的关键措施。在本研究中,两种腙衍生物 N'-[()-呋喃-2-基亚甲基]− 2-(6-甲氧基萘-2-基)丙酰肼 (NFH) 和 2-(6-甲氧基萘-2-) 的性能评估了基)-N'-[()-噻吩-2-基亚甲基]丙酰肼(NTH)在1.0 mol/L HCl溶液中抑制碳钢腐蚀的能力。采用电化学阻抗谱 (EIS)、动电位极化 (PDP) 和扫描电子显微镜与能量色散 X 射线光谱 (SEM-EDX) 来评估化合物的抑制性能。实验结果表明,抑制剂的效率随着浓度的增加而提高。 NFH 达到 90% 的阈值,而 NTH 则达到 93% 的峰值。与 NFH 中的呋喃部分相比,NTH 的这种优异性能归因于其噻吩基团。 PDP 数据显示,这两种化合物充当混合抑制剂,抑制阳极和阴极反应。 EIS 结果表明,由于抑制剂分子的吸附,极化电阻显着增加,同时有效双层电容值降低。抑制剂分子的吸附符合朗缪尔等温线模型,表明与碳钢相互作用的组合物理化学模式,这得到了零电荷电位(PZC)分析的证实。 SEM-EDX 分析证实了噻唑衍生物具有保护金属免受腐蚀的能力。量子化学计算 (QCC) 和分子动力学 (MD) 模拟阐明了反应性描述符和 Fe(110) 表面上的最佳吸附构型。这一探索强调了噻吩部分在增强腙的吸附和腐蚀抑制性能方面的重要性,从而为其未来的精炼和应用提供了战略见解。










































 京公网安备 11010802027423号
京公网安备 11010802027423号