当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overlooked Hydrogen Bond in a Blue Copper Protein Uncovered by Neutron and Sub-Ångström Resolution X-ray Crystallography
Biochemistry ( IF 2.9 ) Pub Date : 2024-01-17 , DOI: 10.1021/acs.biochem.3c00517 Yohta Fukuda 1, 2 , Masami Lintuluoto 3 , Kazuo Kurihara 4 , Kazuya Hasegawa 5 , Tsuyoshi Inoue 1 , Taro Tamada 6, 7
Biochemistry ( IF 2.9 ) Pub Date : 2024-01-17 , DOI: 10.1021/acs.biochem.3c00517 Yohta Fukuda 1, 2 , Masami Lintuluoto 3 , Kazuo Kurihara 4 , Kazuya Hasegawa 5 , Tsuyoshi Inoue 1 , Taro Tamada 6, 7
Affiliation
|
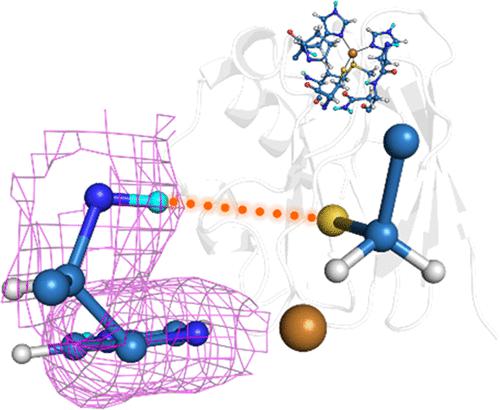
Metalloproteins play fundamental roles in organisms and are utilized as starting points for the directed evolution of artificial enzymes. Knowing the strategies of metalloproteins, by which they exquisitely tune their activities, will not only lead to an understanding of biochemical phenomena but also contribute to various applications. The blue copper protein (BCP) has been a renowned model system to understand the biology, chemistry, and physics of metalloproteins. Pseudoazurin (Paz), a blue copper protein, mediates electron transfer in the bacterial anaerobic respiratory chain. Its redox potential is finely tuned by hydrogen (H) bond networks; however, difficulty in visualizing H atom positions in the protein hinders the detailed understanding of the protein’s structure–function relationship. We here used neutron and sub-ångström resolution X-ray crystallography to directly observe H atoms in Paz. The 0.86-Å-resolution X-ray structure shows that the peptide bond between Pro80 and the His81 Cu ligand deviates from the ideal planar structure. The 1.9-Å-resolution neutron structure confirms a long-overlooked H bond formed by the amide of His81 and the S atom of another Cu ligand Cys78. Quantum mechanics/molecular mechanics calculations show that this H bond increases the redox potential of the Cu site and explains the experimental results well. Our study demonstrates the potential of neutron and sub-ångström resolution X-ray crystallography to understand the chemistry of metalloproteins at atomic and quantum levels.
中文翻译:

中子和亚 Ångström 分辨率 X 射线晶体学发现的蓝铜蛋白中被忽视的氢键
金属蛋白在生物体中起着基本作用,并被用作人工酶定向进化的起点。了解金属蛋白的策略,它们通过这些策略巧妙地调整其活动,不仅有助于理解生化现象,还有助于各种应用。蓝铜蛋白 (BCP) 一直是了解金属蛋白生物学、化学和物理学的著名模型系统。Pseudoazurin (Paz) 是一种蓝铜蛋白,介导细菌厌氧呼吸链中的电子转移。它的氧化还原电位由氢 (H) 键网络微调;然而,难以可视化蛋白质中的 H 原子位置阻碍了对蛋白质结构-功能关系的详细了解。我们在这里使用中子和亚 ångström 分辨率的 X 射线晶体学来直接观察 Paz 中的 H 原子。0.86 Å 分辨率的 X 射线结构表明,Pro80 和 His81 Cu 配体之间的肽键偏离了理想的平面结构。1.9 Å 分辨率的中子结构证实了由 His81 的酰胺和另一个 Cu 配体 Cys78 的 S 原子形成的长期被忽视的 H 键。量子力学/分子力学计算表明,这个 H 键增加了 Cu 位点的氧化还原电位,很好地解释了实验结果。我们的研究证明了中子和亚奥恩斯特伦分辨率 X 射线晶体学在原子和量子水平上了解金属蛋白化学的潜力。
更新日期:2024-01-17
中文翻译:

中子和亚 Ångström 分辨率 X 射线晶体学发现的蓝铜蛋白中被忽视的氢键
金属蛋白在生物体中起着基本作用,并被用作人工酶定向进化的起点。了解金属蛋白的策略,它们通过这些策略巧妙地调整其活动,不仅有助于理解生化现象,还有助于各种应用。蓝铜蛋白 (BCP) 一直是了解金属蛋白生物学、化学和物理学的著名模型系统。Pseudoazurin (Paz) 是一种蓝铜蛋白,介导细菌厌氧呼吸链中的电子转移。它的氧化还原电位由氢 (H) 键网络微调;然而,难以可视化蛋白质中的 H 原子位置阻碍了对蛋白质结构-功能关系的详细了解。我们在这里使用中子和亚 ångström 分辨率的 X 射线晶体学来直接观察 Paz 中的 H 原子。0.86 Å 分辨率的 X 射线结构表明,Pro80 和 His81 Cu 配体之间的肽键偏离了理想的平面结构。1.9 Å 分辨率的中子结构证实了由 His81 的酰胺和另一个 Cu 配体 Cys78 的 S 原子形成的长期被忽视的 H 键。量子力学/分子力学计算表明,这个 H 键增加了 Cu 位点的氧化还原电位,很好地解释了实验结果。我们的研究证明了中子和亚奥恩斯特伦分辨率 X 射线晶体学在原子和量子水平上了解金属蛋白化学的潜力。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号