Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2024-01-19 , DOI: 10.1016/j.molstruc.2023.137417 Shoaib Khan , Rafaqat Hussain , Yousaf Khan , Tayyiaba Iqbal , Hany W. Darwish , Mohamed G.H. Ali
|
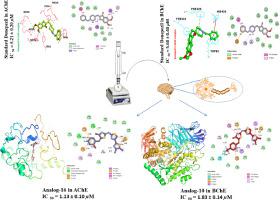
A total of sixteen new derivatives of the bis-thiazole-thiazolidinone skeletal structure were synthesized and examined for their potential as inhibitors of cholinesterase in the context of Alzheimer's disease (AD) therapy. The compounds with the numerical designations 6, 14, and 16 exhibited superior activities in the inhibition of acetylcholinesterase (AChE), while compounds designated as 2, 7, and 10 were found to show remarkable potency against butyrylcholinesterase (BuChE). Furthermore, these drugs had the most minimal IC50 values and demonstrated relative potencies above 50 percent when compared to the efficacious cholinesterase inhibitor, donepezil. The docking experiments performed within the active site of cholinesterase revealed that compounds 6, 14, and 16with their IC50 values 2.81 ± 0.34 μM, 2.45 ± 0.58 μM and 1.33 ± 0.10 μM exhibited favorable binding orientations against AChE, while compounds 2, 7, and 10with their IC50 values of 3.19 ± 0.42 μM, 3.40 ± 0.34 μM and 1.83 ± 0.14 μM displayed comparable binding orientations against BuChE. These compounds demonstrated critical interactions similar to that of donepezil, which can account for their potent cholinesterase activity. It has been shown that the thiazole-thiazolidinone molecule's active site gorge has significant interactions with its amino acid residues. According to the study's results, it is suggested that the novel thiazole-thiazolidinone derivatives, namely compounds 6, 14, and 16 for AChE inhibition and compounds 2, 7, and 10 for BuChE inhibition, have the potential to be used as promising lead compounds in the synthesis of potent thiazole-based thiazolidinone inhibitors targeting these enzymes.
中文翻译:

新型双噻唑-噻唑烷酮杂化衍生物:基于对接研究的合成、结构特性和作为药物竞争者的抗胆碱酯酶生物活性潜力
总共合成了 16 种双噻唑-噻唑烷酮骨架结构的新衍生物,并检查了它们在阿尔茨海默病 (AD) 治疗中作为胆碱酯酶抑制剂的潜力。编号为6、14和16的化合物在抑制乙酰胆碱酯酶(AChE)方面表现出优异的活性,而编号为2、7和10的化合物被发现显示出显着的对抗丁酰胆碱酯酶(BuChE)的效力。此外,与有效的胆碱酯酶抑制剂多奈哌齐相比,这些药物具有最小的 IC 50值,并且显示出高于 50% 的相对效力。在胆碱酯酶活性位点内进行的对接实验表明,化合物6、14和16的IC 50值分别为2.81±0.34μM、2.45±0.58μM和1.33±0.10μM,对AChE表现出良好的结合方向,而化合物2、7则表现出良好的针对AChE的结合方向。和10的 IC 50值为 3.19 ± 0.42 μM、3.40 ± 0.34 μM 和 1.83 ± 0.14 μM,显示出与 BuChE 相当的结合方向。这些化合物表现出与多奈哌齐相似的关键相互作用,这可以解释它们有效的胆碱酯酶活性。研究表明,噻唑-噻唑烷酮分子的活性位点峡谷与其氨基酸残基具有显着的相互作用。根据该研究结果,表明新型噻唑-噻唑烷酮衍生物,即用于抑制AChE的化合物6、14和16以及用于抑制BuChE的化合物2、7和10,有潜力用作有前景的先导化合物合成针对这些酶的有效噻唑基噻唑烷酮抑制剂。















































 京公网安备 11010802027423号
京公网安备 11010802027423号