当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Impact of Oxygen Surface Coverage and Carbidic Carbon on the Activity and Selectivity of Two-Dimensional Molybdenum Carbide (2D-Mo2C) in Fischer–Tropsch Synthesis
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-01-19 , DOI: 10.1021/acscatal.3c03956 Evgenia Kountoupi 1 , Alan J Barrios 2, 3 , Zixuan Chen 1 , Christoph R Müller 1 , Vitaly V Ordomsky 2 , Aleix Comas-Vives 4, 5 , Alexey Fedorov 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-01-19 , DOI: 10.1021/acscatal.3c03956 Evgenia Kountoupi 1 , Alan J Barrios 2, 3 , Zixuan Chen 1 , Christoph R Müller 1 , Vitaly V Ordomsky 2 , Aleix Comas-Vives 4, 5 , Alexey Fedorov 1
Affiliation
|
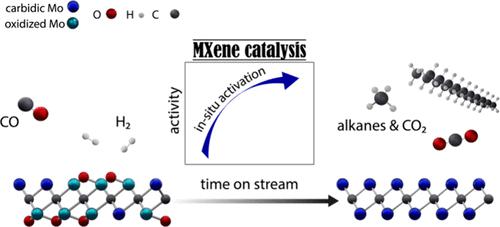
Transformations of oxygenates (CO2, CO, H2O, etc.) via Mo2C-based catalysts are facilitated by the high oxophilicity of the material; however, this can lead to the formation of oxycarbides and complicate the identification of the (most) active catalyst state and active sites. In this context, the two-dimensional (2D) MXene molybdenum carbide Mo2CTx (Tx are passivating surface groups) contains only surface Mo sites and is therefore a highly suitable model catalyst for structure–activity studies. Here, we report that the catalytic activity of Mo2CTx in Fischer–Tropsch (FT) synthesis increases with a decreasing coverage of surface passivating groups (mostly O*). The in situ removal of Tx species and its consequence on CO conversion is highlighted by the observation of a very pronounced activation of Mo2CTx (pretreated in H2 at 400 °C) under FT conditions. This activation process is ascribed to the in situ reductive defunctionalization of Tx groups reaching a catalyst state that is close to 2D-Mo2C (i.e., a material containing no passivating surface groups). Under steady-state FT conditions, 2D-Mo2C yields higher hydrocarbons (C5+ alkanes) with 55% selectivity. Alkanes up to the kerosine range form, with value of α = 0.87, which is ca. twice higher than the α value reported for 3D-Mo2C catalysts. The steady-state productivity of 2D-Mo2C to C5+ hydrocarbons is ca. 2 orders of magnitude higher relative to a reference β-Μo2C catalyst that shows no in situ activation under identical FT conditions. The passivating Tx groups of Mo2CTx can be reductively defunctionalized also by using a higher H2 pretreatment temperature of 500 °C. Yet, this approach leads to a removal of carbidic carbon (as methane), resulting in a 2D-Mo2C1–x catalyst that converts CO to CH4 with 61% selectivity in preference to C5+ hydrocarbons that are formed with only 2% selectivity. Density functional theory (DFT) results attribute the observed selectivity of 2D-Mo2C to C5+ alkanes to a higher energy barrier for the hydrogenation of surface alkyl species relative to the energy barriers for C–C coupling. The removal of O* is the rate-determining step in the FT reaction over 2D-Mo2C, and O* is favorably removed in the form of CO2 relative to H2O, consistent with the observation of a high CO2 selectivity (ca. 50%). The absence of other carbon oxygenates is explained by the energetic favoring of the direct over the hydrogen-assisted dissociative adsorption of CO.
中文翻译:

费托合成中氧表面覆盖度和碳化物碳对二维碳化钼 (2D-Mo2C) 活性和选择性的影响
该材料的高亲氧性促进了含氧化合物(CO 2 、CO、H 2 O等)通过Mo 2 C基催化剂的转化;然而,这可能导致碳氧化物的形成,并使(最)活性催化剂状态和活性位点的识别变得复杂。在这种情况下,二维(2D)MXene碳化钼Mo 2 C T x ( T x是钝化表面基团)仅包含表面Mo位点,因此是非常适合结构-活性研究的模型催化剂。在这里,我们报道了Mo 2 C T x在费托(FT)合成中的催化活性随着表面钝化基团(主要是O*)覆盖度的减少而增加。通过在 FT 条件下观察到 Mo 2 C T x (在 400 °C 的 H 2中预处理)非常明显的活化,突出了T x物质的原位去除及其对 CO 转化的影响。该活化过程归因于T x基团的原位还原去官能化,达到接近2D-Mo 2 C的催化剂状态(即,不含钝化表面基团的材料)。在稳态 FT 条件下,2D-Mo 2 C 产生高级烃(C 5+烷烃),选择性为 55%。烷烃最高可达煤油范围形式,α 值为 0.87,约为 100。比 3D-Mo 2 C 催化剂报道的 α 值高两倍。 2D-Mo 2 C 至C 5+烃的稳态生产率约为。相对于在相同 FT 条件下没有表现出原位活化的参考 β-Mo 2 C 催化剂高 2 个数量级。 Mo 2 C T x的钝化T x基团也可以通过使用500℃的较高H 2预处理温度来还原去官能化。然而,这种方法导致了碳化物碳(如甲烷)的去除,从而产生了 2D-Mo 2 C 1– x催化剂,该催化剂将 CO 转化为 CH 4 ,选择性为 61%,优于仅由 C 5+碳氢化合物形成的碳氢化合物2% 选择性。密度泛函理论 (DFT) 结果将观察到的 2D-Mo 2 C 对 C 5+烷烃的选择性归因于相对于 C-C 偶联能垒而言,表面烷基物种氢化的更高能垒。 O* 的去除是 2D-Mo 2 C FT 反应中的速率决定步骤,相对于 H 2 O,O* 以 CO 2的形式有利地去除,这与观察到的高 CO 2选择性一致(约 50%)。不存在其他碳含氧化合物的原因是直接吸附CO比氢辅助解离吸附更有利于能量。
更新日期:2024-01-19
中文翻译:

费托合成中氧表面覆盖度和碳化物碳对二维碳化钼 (2D-Mo2C) 活性和选择性的影响
该材料的高亲氧性促进了含氧化合物(CO 2 、CO、H 2 O等)通过Mo 2 C基催化剂的转化;然而,这可能导致碳氧化物的形成,并使(最)活性催化剂状态和活性位点的识别变得复杂。在这种情况下,二维(2D)MXene碳化钼Mo 2 C T x ( T x是钝化表面基团)仅包含表面Mo位点,因此是非常适合结构-活性研究的模型催化剂。在这里,我们报道了Mo 2 C T x在费托(FT)合成中的催化活性随着表面钝化基团(主要是O*)覆盖度的减少而增加。通过在 FT 条件下观察到 Mo 2 C T x (在 400 °C 的 H 2中预处理)非常明显的活化,突出了T x物质的原位去除及其对 CO 转化的影响。该活化过程归因于T x基团的原位还原去官能化,达到接近2D-Mo 2 C的催化剂状态(即,不含钝化表面基团的材料)。在稳态 FT 条件下,2D-Mo 2 C 产生高级烃(C 5+烷烃),选择性为 55%。烷烃最高可达煤油范围形式,α 值为 0.87,约为 100。比 3D-Mo 2 C 催化剂报道的 α 值高两倍。 2D-Mo 2 C 至C 5+烃的稳态生产率约为。相对于在相同 FT 条件下没有表现出原位活化的参考 β-Mo 2 C 催化剂高 2 个数量级。 Mo 2 C T x的钝化T x基团也可以通过使用500℃的较高H 2预处理温度来还原去官能化。然而,这种方法导致了碳化物碳(如甲烷)的去除,从而产生了 2D-Mo 2 C 1– x催化剂,该催化剂将 CO 转化为 CH 4 ,选择性为 61%,优于仅由 C 5+碳氢化合物形成的碳氢化合物2% 选择性。密度泛函理论 (DFT) 结果将观察到的 2D-Mo 2 C 对 C 5+烷烃的选择性归因于相对于 C-C 偶联能垒而言,表面烷基物种氢化的更高能垒。 O* 的去除是 2D-Mo 2 C FT 反应中的速率决定步骤,相对于 H 2 O,O* 以 CO 2的形式有利地去除,这与观察到的高 CO 2选择性一致(约 50%)。不存在其他碳含氧化合物的原因是直接吸附CO比氢辅助解离吸附更有利于能量。

































 京公网安备 11010802027423号
京公网安备 11010802027423号