Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical Cross Coupling and Enantioselective Protonation through Asymmetric Photoredox Catalysis
Advanced Science ( IF 14.3 ) Pub Date : 2024-01-17 , DOI: 10.1002/advs.202307773
Manman Kong 1, 2 , Zhuoxi Wang 3 , Xu Ban 2 , Xiaowei Zhao 3 , Yanli Yin 2 , Junmin Zhang 1 , Zhiyong Jiang 1, 2, 3
Advanced Science ( IF 14.3 ) Pub Date : 2024-01-17 , DOI: 10.1002/advs.202307773
Manman Kong 1, 2 , Zhuoxi Wang 3 , Xu Ban 2 , Xiaowei Zhao 3 , Yanli Yin 2 , Junmin Zhang 1 , Zhiyong Jiang 1, 2, 3
Affiliation
|
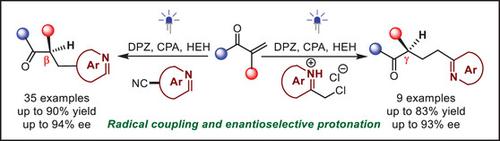
An unprecedented enantioselective protonation reaction enabled by photoredox catalytic radical coupling is developed. Under cooperative dicynopyrazine-derived chromophore (DPZ) as a photosensitizer and a chiral phosphoric acid catalyst, and Hantzsch ester as a sacrificial reductant, the transformations between α-substituted enones and cyanoazaarenes or 2-(chloromethyl)azaaren-1-iums can proceed a tandem reduction, radical coupling, and enantioselective protonation process efficiently. Two classes of pharmaceutically important enantioenriched azaarene variants, which contain a synthetically versatile ketone-substituted tertiary carbon stereocenter at the β- or γ-position of the azaarenes, are synthesized with high yields and ees.
中文翻译:

通过不对称光氧化还原催化进行自由基交叉偶联和对映选择性质子化
开发了一种前所未有的通过光氧化还原催化自由基偶联实现的对映选择性质子化反应。在二氰基吡嗪衍生的发色团(DPZ)作为光敏剂和手性磷酸催化剂以及Hantzsch酯作为牺牲还原剂的协同作用下,α-取代烯酮与氰基氮杂芳烃或2-(氯甲基)氮杂芳烃-1-iums之间的转化可以进行高效的串联还原、自由基偶联和对映选择性质子化过程。两类药学上重要的对映体富集氮杂芳烃变体,在氮杂芳烃的β或γ位置含有合成通用的酮取代的叔碳立构中心,以高产率和 ee 合成。
更新日期:2024-01-17
中文翻译:

通过不对称光氧化还原催化进行自由基交叉偶联和对映选择性质子化
开发了一种前所未有的通过光氧化还原催化自由基偶联实现的对映选择性质子化反应。在二氰基吡嗪衍生的发色团(DPZ)作为光敏剂和手性磷酸催化剂以及Hantzsch酯作为牺牲还原剂的协同作用下,α-取代烯酮与氰基氮杂芳烃或2-(氯甲基)氮杂芳烃-1-iums之间的转化可以进行高效的串联还原、自由基偶联和对映选择性质子化过程。两类药学上重要的对映体富集氮杂芳烃变体,在氮杂芳烃的β或γ位置含有合成通用的酮取代的叔碳立构中心,以高产率和 ee 合成。

































 京公网安备 11010802027423号
京公网安备 11010802027423号