SynOpen ( IF 2.0 ) Pub Date : 2024-01-17 , DOI: 10.1055/a-2236-0803
Sambasivarao Kotha 1 , Ramakrishna Reddy Keesari 1
|
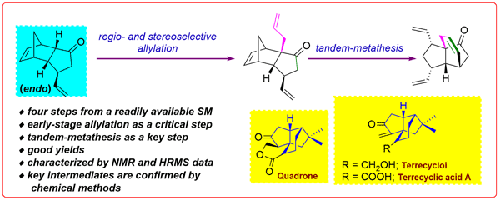
We disclose a useful synthetic method for preparing the propellane-type 5/5/6-tricyclic system that is present in diquinane-based natural products such as quadrone, terrecyclic acid A, or terrecyclol. The method involves an LDA-mediated regio- and stereoselective allylation and a tandem metathesis as key steps. The target molecules were assembled in just two steps starting from a readily available building block, a 3β-vinyl tricyclic ketone prepared from endo-dicyclopentadiene-1-one. All the compounds prepared were characterized by NMR analyses and/or chemical methods. The synthetic methods demonstrated here are useful in syntheses of quadranoid-type natural products.
中文翻译:

通过串联复分解合成丙烷型 5/5/6-三环系统:四元骨架的新方法
我们公开了一种用于制备丙烷型5/5/6-三环系统的有用合成方法,该系统存在于基于二奎烷的天然产物例如四氢醌、三环酸A或特瑞环醇中。该方法涉及 LDA 介导的区域选择性和立体选择性烯丙基化以及串联复分解作为关键步骤。从易于获得的结构单元(由内二环戊二烯-1-酮制备的 3β-乙烯基三环酮)开始,只需两步即可组装目标分子。所有制备的化合物均通过 NMR 分析和/或化学方法进行表征。这里展示的合成方法可用于四烷类天然产物的合成。

































 京公网安备 11010802027423号
京公网安备 11010802027423号