当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Demonstrating on-demand production of bio-ethylene oxide in a two-step dehydration-epoxidation process with chemical looping operations
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-01-17 , DOI: 10.1016/j.cej.2024.148804 Joseph C. Gebers , Ewa J. Marek
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-01-17 , DOI: 10.1016/j.cej.2024.148804 Joseph C. Gebers , Ewa J. Marek
|
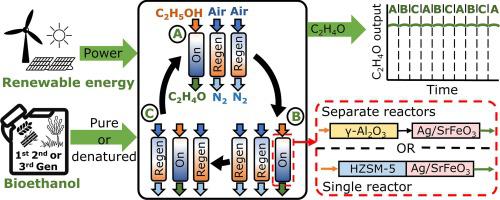
Ethylene oxide (EO) is a key chemical intermediate produced almost exclusively from petrochemically derived ethylene. Currently, EO manufacturing involves epoxidation of ethylene with O2 over Ag/Al2 O3 - one of the highest CO2 -emitting processes in the chemical sector (Boulamanti and Moya, 2017). The flammability hazards associated with incumbent methods and sluggish process start-ups prevent small-scale, flexible operations or alignment with intermittently available renewable resources. Presented herein is a novel process for on-demand production of bio-EO from bioethanol. Ethanol, laboratory-grade or denatured, was first dehydrated to ethylene over HZSM-5 (at 280 °C) or γ-Al2 O3 (at 350 °C) catalysts, producing ethylene and water. The ethylene stream was then selectively oxidised to EO over Ag/SrFeO3 at 270 °C using lattice oxygen from a solid – SrFeO3 , employed to drive chemical looping epoxidation (CLE). For a configuration where the dehydration and epoxidation reactions were carried out in separate reactors, the process produced EO with 57 % selectivity at 15 % conversion of ethylene, thus exceeding the incumbent approach with pure Ag/α-Al2 O3 and O2. In an alternative configuration, experiments were carried out in one dehydration-epoxidation reactor layered with two catalysts: HZSM-5 and Ag/SrFeO3 . The results revealed that water presence at a percentage level enhanced unselective combustion. In-situ removal of water, possible with an additional layer of a drying material between the two catalysts, proved effective in boosting the process performance, reaching selectivity to EO of 50 % at 12 % conversion of ethylene. The catalysts showed no sign of deactivation when using denatured ethanol or when performing experiments intermittently. Hence, our novel process can be kept offline without penalty, allowing for on demand production and complete alignment with renewable resources.
中文翻译:

展示通过化学循环操作的两步脱水-环氧化工艺按需生产生物环氧乙烷
环氧乙烷 (EO) 是一种关键的化学中间体,几乎完全由石化衍生的乙烯生产。目前,环氧乙烷生产涉及乙烯与 O2 在 Ag/Al2O3 上的环氧化反应,这是化学行业二氧化碳排放量最高的工艺之一(Boulamanti 和 Moya,2017)。与现有方法和缓慢的工艺启动相关的易燃危险阻碍了小规模、灵活的操作或与间歇性可用的可再生资源的结合。本文提出了一种从生物乙醇按需生产生物环氧乙烷的新工艺。实验室级或变性乙醇首先在 HZSM-5(280 °C)或 γ-Al2O3(350 °C)催化剂上脱水为乙烯,产生乙烯和水。然后,在 270 °C 下,使用固体 SrFeO3 中的晶格氧,通过 Ag/SrFeO3 将乙烯流选择性氧化为 EO,用于驱动化学循环环氧化 (CLE)。对于脱水和环氧化反应在单独的反应器中进行的配置,该工艺以 57% 的选择性、15% 的乙烯转化率生产 EO,从而超过了使用纯 Ag/α-Al2O3 和 O2 的现有方法。在另一种配置中,实验在一个分层有两种催化剂的脱水环氧化反应器中进行:HZSM-5 和 Ag/SrFeO3。结果表明,一定百分比水平的水存在增强了非选择性燃烧。事实证明,可以通过在两种催化剂之间添加一层干燥材料来原位去除水,这可以有效提高工艺性能,在乙烯转化率为 12% 时,EO 选择性达到 50%。当使用工业乙醇或间歇进行实验时,催化剂没有表现出失活的迹象。 因此,我们的新颖工艺可以保持离线状态而不会受到惩罚,从而实现按需生产并与可再生资源完全一致。
更新日期:2024-01-17
中文翻译:

展示通过化学循环操作的两步脱水-环氧化工艺按需生产生物环氧乙烷
环氧乙烷 (EO) 是一种关键的化学中间体,几乎完全由石化衍生的乙烯生产。目前,环氧乙烷生产涉及乙烯与 O2 在 Ag/Al2O3 上的环氧化反应,这是化学行业二氧化碳排放量最高的工艺之一(Boulamanti 和 Moya,2017)。与现有方法和缓慢的工艺启动相关的易燃危险阻碍了小规模、灵活的操作或与间歇性可用的可再生资源的结合。本文提出了一种从生物乙醇按需生产生物环氧乙烷的新工艺。实验室级或变性乙醇首先在 HZSM-5(280 °C)或 γ-Al2O3(350 °C)催化剂上脱水为乙烯,产生乙烯和水。然后,在 270 °C 下,使用固体 SrFeO3 中的晶格氧,通过 Ag/SrFeO3 将乙烯流选择性氧化为 EO,用于驱动化学循环环氧化 (CLE)。对于脱水和环氧化反应在单独的反应器中进行的配置,该工艺以 57% 的选择性、15% 的乙烯转化率生产 EO,从而超过了使用纯 Ag/α-Al2O3 和 O2 的现有方法。在另一种配置中,实验在一个分层有两种催化剂的脱水环氧化反应器中进行:HZSM-5 和 Ag/SrFeO3。结果表明,一定百分比水平的水存在增强了非选择性燃烧。事实证明,可以通过在两种催化剂之间添加一层干燥材料来原位去除水,这可以有效提高工艺性能,在乙烯转化率为 12% 时,EO 选择性达到 50%。当使用工业乙醇或间歇进行实验时,催化剂没有表现出失活的迹象。 因此,我们的新颖工艺可以保持离线状态而不会受到惩罚,从而实现按需生产并与可再生资源完全一致。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号