当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In silico Design, Synthesis and Biological Evaluation of Novel Thieno[3,2-d]pyrimidine Derivatives for Cancer Therapy – A Preliminary Study on the Inhibitory Potential towards ATR Kinase Domain and PIKK Family
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2024-01-17 , DOI: 10.1002/cbdv.202302071 Samvel N Sirakanyan 1 , Haritha Dilip 2 , Athina Geronikaki 3 , Domenico Spinelli 4 , Sivapriya Kirubakaran 2 , Anthi Petrou 3 , Elmira K Hakobyan 1 , Victor G Kartsev 5 , Ervand G Paronikyan 1 , Hasmik A Yegoryan 1 , Lilit V Yermalovyan 1 , Anush A Hovakimyan 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2024-01-17 , DOI: 10.1002/cbdv.202302071 Samvel N Sirakanyan 1 , Haritha Dilip 2 , Athina Geronikaki 3 , Domenico Spinelli 4 , Sivapriya Kirubakaran 2 , Anthi Petrou 3 , Elmira K Hakobyan 1 , Victor G Kartsev 5 , Ervand G Paronikyan 1 , Hasmik A Yegoryan 1 , Lilit V Yermalovyan 1 , Anush A Hovakimyan 1
Affiliation
|
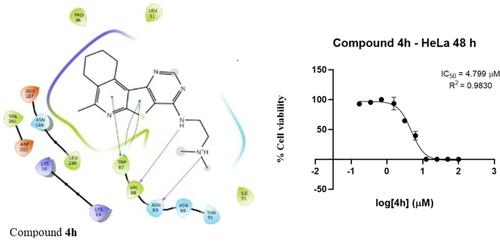
Continuing our studies in the field of new heterocyclic compounds with biological interest, herein we report the synthesis and anticancer activity of new N- and S-substituted derivatives of tetracyclic pyrido[3′,2′ : 4,5]thieno[3,2-d]pyrimidines. In this regard, starting from the thieno[2,3-b]pyridine-2-carboxylates, the corresponding 8(9)-aminopyrido[3′,2′ : 4,5]thieno[3,2-d]pyrimidin-7(8)-ones, as well as chloro derivatives were obtained. Based on the latter, amino, hydrazino and S-alkyl derivatives of pyrido[3′,2′ : 4,5]thieno[3,2-d]pyrimidines were synthesized subsequently. The current study focuses on identifying the potential of thieno[3,2-d]pyrimidine derivatives primarily towards ATR kinase inhibition, through computational predictions, followed by synthesis and cancer cell viability studies, along with an aim to develop the core as PIKK inhibitors for cancer therapy.
中文翻译:

用于癌症治疗的新型噻吩并[3,2-d]嘧啶衍生物的计算机设计、合成和生物学评价——对 ATR 激酶结构域和 PIKK 家族抑制潜力的初步研究
继续我们在具有生物学意义的新型杂环化合物领域的研究,本文报道了新型四环吡啶并[3',2' : 4,5]噻吩并[3,2]的N-和S-取代衍生物的合成和抗癌活性-d ]嘧啶。在这方面,从噻吩并[2,3- b ]吡啶-2-羧酸盐开始,相应的8(9)-氨基吡啶并[3',2':4,5]噻吩并[3,2- d ]嘧啶-获得了7(8)-酮以及氯代衍生物。在此基础上,随后合成了吡啶并[3',2':4,5]噻吩并[3,2- d ]嘧啶的氨基、肼基和S-烷基衍生物。目前的研究重点是通过计算预测确定噻吩并[3,2- d ]嘧啶衍生物主要抑制 ATR 激酶的潜力,然后进行合成和癌细胞活力研究,并旨在开发作为 PIKK 抑制剂的核心癌症治疗。
更新日期:2024-01-17
中文翻译:

用于癌症治疗的新型噻吩并[3,2-d]嘧啶衍生物的计算机设计、合成和生物学评价——对 ATR 激酶结构域和 PIKK 家族抑制潜力的初步研究
继续我们在具有生物学意义的新型杂环化合物领域的研究,本文报道了新型四环吡啶并[3',2' : 4,5]噻吩并[3,2]的N-和S-取代衍生物的合成和抗癌活性-d ]嘧啶。在这方面,从噻吩并[2,3- b ]吡啶-2-羧酸盐开始,相应的8(9)-氨基吡啶并[3',2':4,5]噻吩并[3,2- d ]嘧啶-获得了7(8)-酮以及氯代衍生物。在此基础上,随后合成了吡啶并[3',2':4,5]噻吩并[3,2- d ]嘧啶的氨基、肼基和S-烷基衍生物。目前的研究重点是通过计算预测确定噻吩并[3,2- d ]嘧啶衍生物主要抑制 ATR 激酶的潜力,然后进行合成和癌细胞活力研究,并旨在开发作为 PIKK 抑制剂的核心癌症治疗。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号