当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deciphering the Kinetics of Spontaneous Generation of H2O2 in Individual Water Microdroplets
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-17 , DOI: 10.1021/jacs.3c09864
Kai Zhou 1 , Hua Su 2 , Jia Gao 1 , Haoran Li 1 , Shasha Liu 1 , Xuannuo Yi 1 , Zhibing Zhang 3 , Wei Wang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-17 , DOI: 10.1021/jacs.3c09864
Kai Zhou 1 , Hua Su 2 , Jia Gao 1 , Haoran Li 1 , Shasha Liu 1 , Xuannuo Yi 1 , Zhibing Zhang 3 , Wei Wang 1
Affiliation
|
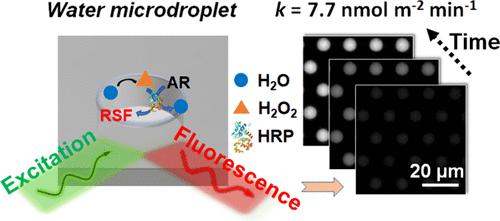
Spontaneous generation of H2O2 in sub-10 μm-sized water microdroplets has received increasing interest since its first discovery in 2019. On the other hand, due to the short lifetime of these microdroplets (rapid evaporation) and lack of suitable tools to real-time monitor the generation of H2O2 in individual microdroplets, such a seemingly thermodynamically unfavorable process has also raised vigorous debates on the origin of H2O2 and the underlying mechanism. Herein, we prepared water microdroplets with a long lifetime (>1 h) by virtue of microwell confinement and dynamically monitored the spontaneous generation of H2O2 in individual microdroplets via time-lapsed fluorescence imaging. It was unveiled that H2O2 was continuously generated in the as-prepared water microdroplets and an apparent equilibrium concentration of ∼3 μM of H2O2 in the presence of a H2O2-consuming reaction can be obtained. Through engineering the geometry of these microdroplets, we further revealed that the generation rates of H2O2 in individual microdroplets were positively proportional to their surface-to-volume ratios. This also allowed us to extract a maximal H2O2 generation rate of 7.7 nmol m–2 min–1 in the presence of a H2O2-consuming reaction and derive the corresponding probability of spontaneous conversion of interfacial H2O into H2O2 for the first time, that is, ∼1 of 65,000 water molecules in 1 s. These findings delivered strong evidence that the spontaneous generation of H2O2 indeed occurs at the surface of microdroplets and provided us with an important starting point to further enhance the yield of H2O2 in water microdroplets for future applications.
中文翻译:

破译单个水微滴中自发生成 H2O2 的动力学
自 2019 年首次发现以来,在亚 10 μm 尺寸的水微滴中自发生成 H 2 O 2受到了越来越多的关注。另一方面,由于这些微滴的寿命短(快速蒸发)并且缺乏合适的工具来生成 H 2 O 2 。实时监测单个微滴中H 2 O 2的生成,这种看似热力学上不利的过程也引发了关于H 2 O 2的起源及其潜在机制的激烈争论。在此,我们通过微孔限制制备了具有长寿命(>1 h)的水微滴,并通过延时荧光成像动态监测单个微滴中H 2 O 2的自发生成。结果表明,在所制备的水微滴中连续产生H 2 O 2 ,并且在存在H 2 O 2消耗反应的情况下可以获得~3μM的H 2 O 2表观平衡浓度。通过设计这些微滴的几何形状,我们进一步揭示了单个微滴中 H 2 O 2的生成速率与其表面积与体积比成正比。这也使我们能够提取最大 H 2 O 2生成率为 7。7 nmol m –2 min –1在存在 H 2 O 2消耗反应的情况下,并首次推导出界面 H 2 O 自发转化为 H 2 O 2的相应概率,即 ∼1 of 65,000 1秒内水分子。这些发现有力地证明了H 2 O 2的自发生成确实发生在微滴表面,并为我们进一步提高水微滴中H 2 O 2的产率以供未来应用提供了重要的起点。
更新日期:2024-01-17
中文翻译:

破译单个水微滴中自发生成 H2O2 的动力学
自 2019 年首次发现以来,在亚 10 μm 尺寸的水微滴中自发生成 H 2 O 2受到了越来越多的关注。另一方面,由于这些微滴的寿命短(快速蒸发)并且缺乏合适的工具来生成 H 2 O 2 。实时监测单个微滴中H 2 O 2的生成,这种看似热力学上不利的过程也引发了关于H 2 O 2的起源及其潜在机制的激烈争论。在此,我们通过微孔限制制备了具有长寿命(>1 h)的水微滴,并通过延时荧光成像动态监测单个微滴中H 2 O 2的自发生成。结果表明,在所制备的水微滴中连续产生H 2 O 2 ,并且在存在H 2 O 2消耗反应的情况下可以获得~3μM的H 2 O 2表观平衡浓度。通过设计这些微滴的几何形状,我们进一步揭示了单个微滴中 H 2 O 2的生成速率与其表面积与体积比成正比。这也使我们能够提取最大 H 2 O 2生成率为 7。7 nmol m –2 min –1在存在 H 2 O 2消耗反应的情况下,并首次推导出界面 H 2 O 自发转化为 H 2 O 2的相应概率,即 ∼1 of 65,000 1秒内水分子。这些发现有力地证明了H 2 O 2的自发生成确实发生在微滴表面,并为我们进一步提高水微滴中H 2 O 2的产率以供未来应用提供了重要的起点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号