当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biomimetic Synthesis and Chemical Proteomics Reveal the Mechanism of Action and Functional Targets of Phloroglucinol Meroterpenoids
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-17 , DOI: 10.1021/jacs.3c10741 Amy K Bracken 1 , Colby E Gekko 1 , Nina O Suss 1 , Emma E Lueders 1 , Qi Cui 1 , Qin Fu 2 , Andy C W Lui 2 , Elizabeth T Anderson 2 , Sheng Zhang 2 , Mikail E Abbasov
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-17 , DOI: 10.1021/jacs.3c10741 Amy K Bracken 1 , Colby E Gekko 1 , Nina O Suss 1 , Emma E Lueders 1 , Qi Cui 1 , Qin Fu 2 , Andy C W Lui 2 , Elizabeth T Anderson 2 , Sheng Zhang 2 , Mikail E Abbasov
Affiliation
|
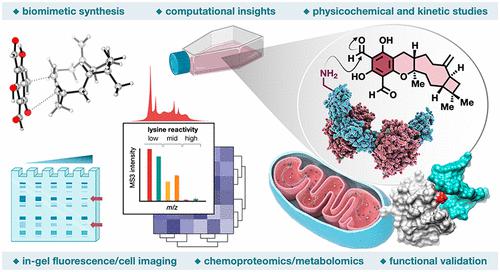
Natural products perennially serve as prolific sources of drug leads and chemical probes, fueling the development of numerous therapeutics. Despite their scarcity, natural products that modulate protein function through covalent interactions with lysine residues hold immense potential to unlock new therapeutic interventions and advance our understanding of the biological processes governed by these modifications. Phloroglucinol meroterpenoids constitute one of the most expansive classes of natural products, displaying a plethora of biological activities. However, their mechanism of action and cellular targets have, until now, remained elusive. In this study, we detail the concise biomimetic synthesis, computational mechanistic insights, physicochemical attributes, kinetic parameters, molecular mechanism of action, and functional cellular targets of several phloroglucinol meroterpenoids. We harness synthetic clickable analogues of natural products to probe their disparate proteome-wide reactivity and subcellular localization through in-gel fluorescence scanning and cell imaging. By implementing sample multiplexing and a redesigned lysine-targeting probe, we streamline a quantitative activity-based protein profiling, enabling the direct mapping of global reactivity and ligandability of proteinaceous lysines in human cells. Leveraging this framework, we identify numerous lysine–meroterpenoid interactions in breast cancer cells at tractable protein sites across diverse structural and functional classes, including those historically deemed undruggable. We validate that phloroglucinol meroterpenoids perturb biochemical functions through stereoselective and site-specific modification of lysines in proteins vital for breast cancer metabolism, including lipid signaling, mitochondrial respiration, and glycolysis. These findings underscore the broad potential of phloroglucinol meroterpenoids for targeting functional lysines in the human proteome.
中文翻译:

仿生合成和化学蛋白质组学揭示了间苯三酚类鼻萜的作用机制和功能靶点
天然产物常年成为药物先导物和化学探针的多产来源,推动了许多治疗方法的发展。尽管它们稀缺,但通过与赖氨酸残基的共价相互作用调节蛋白质功能的天然产物具有巨大的潜力,可以解锁新的治疗干预措施,并促进我们对受这些修饰控制的生物过程的理解。间苯三酚硫萜类化合物是最广泛的天然产物类别之一,显示出大量的生物活性。然而,直到现在,它们的作用机制和细胞靶标仍然难以捉摸。在这项研究中,我们详细介绍了几种间苯三酚甲萜类化合物的简明仿生合成、计算机制、物理化学属性、动力学参数、分子作用机制和功能性细胞靶标。我们利用天然产物的合成可点击类似物,通过凝胶内荧光扫描和细胞成像来探测它们不同的蛋白质组范围反应性和亚细胞定位。通过实施样品多路复用和重新设计的赖氨酸靶向探针,我们简化了基于定量活性的蛋白质分析,从而能够直接绘制人类细胞中蛋白质赖氨酸的整体反应性和配体性。利用这个框架,我们在乳腺癌细胞中可处理的蛋白质位点确定了许多赖氨酸-甲萜类化合物相互作用,涉及不同的结构和功能类别,包括那些历史上被认为不可成药的。 我们验证了间苯三酚甲萜类化合物通过对乳腺癌代谢至关重要的蛋白质中赖氨酸的立体选择性和位点特异性修饰来扰乱生化功能,包括脂质信号传导、线粒体呼吸和糖酵解。这些发现强调了间苯三酚甲萜类化合物在靶向人类蛋白质组中功能性赖氨酸方面的广泛潜力。
更新日期:2024-01-17
中文翻译:

仿生合成和化学蛋白质组学揭示了间苯三酚类鼻萜的作用机制和功能靶点
天然产物常年成为药物先导物和化学探针的多产来源,推动了许多治疗方法的发展。尽管它们稀缺,但通过与赖氨酸残基的共价相互作用调节蛋白质功能的天然产物具有巨大的潜力,可以解锁新的治疗干预措施,并促进我们对受这些修饰控制的生物过程的理解。间苯三酚硫萜类化合物是最广泛的天然产物类别之一,显示出大量的生物活性。然而,直到现在,它们的作用机制和细胞靶标仍然难以捉摸。在这项研究中,我们详细介绍了几种间苯三酚甲萜类化合物的简明仿生合成、计算机制、物理化学属性、动力学参数、分子作用机制和功能性细胞靶标。我们利用天然产物的合成可点击类似物,通过凝胶内荧光扫描和细胞成像来探测它们不同的蛋白质组范围反应性和亚细胞定位。通过实施样品多路复用和重新设计的赖氨酸靶向探针,我们简化了基于定量活性的蛋白质分析,从而能够直接绘制人类细胞中蛋白质赖氨酸的整体反应性和配体性。利用这个框架,我们在乳腺癌细胞中可处理的蛋白质位点确定了许多赖氨酸-甲萜类化合物相互作用,涉及不同的结构和功能类别,包括那些历史上被认为不可成药的。 我们验证了间苯三酚甲萜类化合物通过对乳腺癌代谢至关重要的蛋白质中赖氨酸的立体选择性和位点特异性修饰来扰乱生化功能,包括脂质信号传导、线粒体呼吸和糖酵解。这些发现强调了间苯三酚甲萜类化合物在靶向人类蛋白质组中功能性赖氨酸方面的广泛潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号