当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cation Modifies Interfacial Water Structures on Platinum during Alkaline Hydrogen Electrocatalysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-16 , DOI: 10.1021/jacs.3c09128 Pengtao Xu 1 , Ruiyu Wang 2, 3 , Haojian Zhang 1 , Vincenzo Carnevale 4, 5 , Eric Borguet 2, 3 , Jin Suntivich 1, 6
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-16 , DOI: 10.1021/jacs.3c09128 Pengtao Xu 1 , Ruiyu Wang 2, 3 , Haojian Zhang 1 , Vincenzo Carnevale 4, 5 , Eric Borguet 2, 3 , Jin Suntivich 1, 6
Affiliation
|
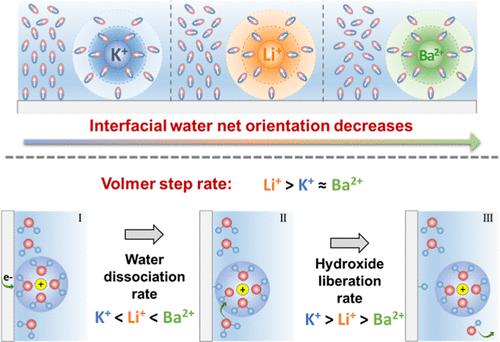
The molecular details of an electrocatalytic interface play an essential role in the production of sustainable fuels and value-added chemicals. Many electrochemical reactions exhibit strong cation-dependent activities, but how cations affect reaction kinetics is still elusive. We report the effect of cations (K+, Li+, and Ba2+) on the interfacial water structure using second-harmonic generation (SHG) and classical molecular dynamics (MD) simulation. The second- (χH2O(2)) and third-order (χH2O(3)) optical susceptibilities of water on Pt are smaller in the presence of Ba2+ compared to those of K+, suggesting that cations can affect the interfacial water orientation. MD simulation reproduces experimental SHG observations and further shows that the competition between cation hydration and interfacial water alignment governs the net water orientation. The impact of cations on interfacial water supports a cation hydration-mediated mechanism for hydrogen electrocatalysis; i.e., the reaction occurs via water dissociation followed by cation-assisted hydroxide/water exchange on Pt. Our study highlights the role of interfacial water in electrocatalysis and how innocent additives (such as cations) can affect the local electrochemical environment.
中文翻译:

碱性氢电催化过程中阳离子改变铂上的界面水结构
电催化界面的分子细节在可持续燃料和增值化学品的生产中发挥着重要作用。许多电化学反应表现出强烈的阳离子依赖性活性,但阳离子如何影响反应动力学仍然难以捉摸。我们使用二次谐波生成 (SHG) 和经典分子动力学 (MD) 模拟报告了阳离子(K + 、Li +和 Ba 2+ )对界面水结构的影响。 Ba 2+存在时,水在 Pt 上的二阶 (χ H 2 O (2) ) 和三阶 (χ H 2 O (3) ) 光学磁化率比 K +更小,这表明阳离子会影响界面水的取向。 MD模拟再现了实验SHG观察结果,并进一步表明阳离子水合和界面水排列之间的竞争控制着净水方向。阳离子对界面水的影响支持了氢电催化的阳离子水合介导机制;即,反应通过水离解发生,然后在 Pt 上进行阳离子辅助的氢氧化物/水交换。我们的研究强调了界面水在电催化中的作用以及无害添加剂(例如阳离子)如何影响局部电化学环境。
更新日期:2024-01-16
中文翻译:

碱性氢电催化过程中阳离子改变铂上的界面水结构
电催化界面的分子细节在可持续燃料和增值化学品的生产中发挥着重要作用。许多电化学反应表现出强烈的阳离子依赖性活性,但阳离子如何影响反应动力学仍然难以捉摸。我们使用二次谐波生成 (SHG) 和经典分子动力学 (MD) 模拟报告了阳离子(K + 、Li +和 Ba 2+ )对界面水结构的影响。 Ba 2+存在时,水在 Pt 上的二阶 (χ H 2 O (2) ) 和三阶 (χ H 2 O (3) ) 光学磁化率比 K +更小,这表明阳离子会影响界面水的取向。 MD模拟再现了实验SHG观察结果,并进一步表明阳离子水合和界面水排列之间的竞争控制着净水方向。阳离子对界面水的影响支持了氢电催化的阳离子水合介导机制;即,反应通过水离解发生,然后在 Pt 上进行阳离子辅助的氢氧化物/水交换。我们的研究强调了界面水在电催化中的作用以及无害添加剂(例如阳离子)如何影响局部电化学环境。






























 京公网安备 11010802027423号
京公网安备 11010802027423号