当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 15N-Pyridines and Higher Mass Isotopologs via Zincke Imine Intermediates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-16 , DOI: 10.1021/jacs.3c12445 Hillary M H Nguyen 1 , David C Thomas 1 , Marie A Hart 1 , Kaila R Steenback 1 , Jeffrey N Levy 1 , Andrew McNally 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-16 , DOI: 10.1021/jacs.3c12445 Hillary M H Nguyen 1 , David C Thomas 1 , Marie A Hart 1 , Kaila R Steenback 1 , Jeffrey N Levy 1 , Andrew McNally 1
Affiliation
|
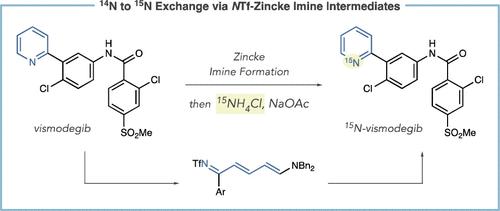
Methods to incorporate stable radioisotopes are integral to pharmaceutical and agrochemical development. However, despite the prevalence of pyridines in candidate compounds, methods to incorporate 15N atoms within their structures are limited. Here, we present a general approach to pyridine 15N-labeling that proceeds via ring-opening to NTf-Zincke imines and then ring-closure with commercially available 15NH4Cl salts. This process functions on a range of substituted pyridines, from simple building block-type compounds to late-stage labeling of complex pharmaceuticals, and 15N-incorporation is >95% in most cases. The reactivity of the Zincke imine intermediates also enables deuteration of the pyridine C3- and C5-positions, resulting in higher mass isotopologs required for LCMS analysis of biological fluids during drug development.
中文翻译:

通过锌亚胺中间体合成 15N-吡啶和高质量同位素
掺入稳定放射性同位素的方法是制药和农用化学品开发不可或缺的一部分。然而,尽管吡啶在候选化合物中普遍存在,但在其结构中掺入 15个 N 原子的方法是有限的。在这里,我们提出了一种吡啶 15N 标记的通用方法,该方法通过开环到 NTf-Zincke 亚胺,然后用市售的 15NH4Cl 盐闭合。该工艺适用于一系列取代的吡啶,从简单的构建块型化合物到复杂药物的后期标记,在大多数情况下,15N 掺入率为 >95%。锌亚胺中间体的反应性还能够使吡啶 C3 和 C5 位点的氘化,从而在药物开发过程中对生物液体进行 LCMS 分析所需的高质量同位素。
更新日期:2024-01-16
中文翻译:

通过锌亚胺中间体合成 15N-吡啶和高质量同位素
掺入稳定放射性同位素的方法是制药和农用化学品开发不可或缺的一部分。然而,尽管吡啶在候选化合物中普遍存在,但在其结构中掺入 15个 N 原子的方法是有限的。在这里,我们提出了一种吡啶 15N 标记的通用方法,该方法通过开环到 NTf-Zincke 亚胺,然后用市售的 15NH4Cl 盐闭合。该工艺适用于一系列取代的吡啶,从简单的构建块型化合物到复杂药物的后期标记,在大多数情况下,15N 掺入率为 >95%。锌亚胺中间体的反应性还能够使吡啶 C3 和 C5 位点的氘化,从而在药物开发过程中对生物液体进行 LCMS 分析所需的高质量同位素。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号