当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Aziridine-Forming α-Ketoglutarate-Dependent Oxygenase in l-Isovaline Biosynthesis
Organic Letters ( IF 4.9 ) Pub Date : 2024-01-16 , DOI: 10.1021/acs.orglett.3c04185 Lu Zhou 1, 2 , Takayoshi Awakawa 1, 2 , Richiro Ushimaru 1, 3 , Masahiro Kanaida 1 , Ikuro Abe 1, 3
Organic Letters ( IF 4.9 ) Pub Date : 2024-01-16 , DOI: 10.1021/acs.orglett.3c04185 Lu Zhou 1, 2 , Takayoshi Awakawa 1, 2 , Richiro Ushimaru 1, 3 , Masahiro Kanaida 1 , Ikuro Abe 1, 3
Affiliation
|
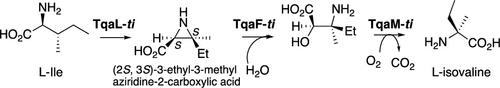
l-Isovaline biosynthesis by TqaLFM-ti from Tolypocladium inflatum was demonstrated in vitro. The biochemical analysis of the α-ketoglutarate-dependent oxygenase TqaL-ti revealed that it produces (2S,3S)-3-ethyl-3-methylaziridine-2-carboxylic acid from l-isoleucine, thus exhibiting a stereoselectivity different from those of the reported homologues. Remarkably, a single mutation on I295 in TqaL-ti completely exchanged its stereoselectivity to produce the C-3 stereoisomer. TqaFM-ti generates d-isovaline from (2S,3R)-aziridine-2-carboxylic acid, suggesting that the stereochemistry of the TqaL product defines that of isovaline.
中文翻译:

L-异缬氨酸生物合成中氮丙啶形成 α-酮戊二酸依赖性加氧酶的表征
体外证明了Tolypocladium inflatum中 TqaLFM -ti的l-异缬氨酸生物合成。对α-酮戊二酸依赖性加氧酶TqaL -ti的生化分析表明,它从l-异亮氨酸产生( 2S , 3S )-3-乙基-3-甲基氮丙啶-2-羧酸,从而表现出与那些不同的立体选择性。所报道的同系物。值得注意的是,TqaL- ti中 I295 的单个突变完全改变了其立体选择性,产生了 C-3 立体异构体。 TqaFM- ti从 (2 S ,3 R )-氮丙啶-2-羧酸生成d -异缬氨酸,这表明 TqaL 产物的立体化学定义了异缬氨酸的立体化学。
更新日期:2024-01-16
中文翻译:

L-异缬氨酸生物合成中氮丙啶形成 α-酮戊二酸依赖性加氧酶的表征
体外证明了Tolypocladium inflatum中 TqaLFM -ti的l-异缬氨酸生物合成。对α-酮戊二酸依赖性加氧酶TqaL -ti的生化分析表明,它从l-异亮氨酸产生( 2S , 3S )-3-乙基-3-甲基氮丙啶-2-羧酸,从而表现出与那些不同的立体选择性。所报道的同系物。值得注意的是,TqaL- ti中 I295 的单个突变完全改变了其立体选择性,产生了 C-3 立体异构体。 TqaFM- ti从 (2 S ,3 R )-氮丙啶-2-羧酸生成d -异缬氨酸,这表明 TqaL 产物的立体化学定义了异缬氨酸的立体化学。






























 京公网安备 11010802027423号
京公网安备 11010802027423号