当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Reactivity of 1,2,3-Benzotriazin-4(3H)-ones: Photocatalytic Synthesis of 3-Substituted Isoindolinones Achieved through a Nitrogen-Mediated Hydrogen Atom Shift
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-01-16 , DOI: 10.1021/acs.joc.3c02545 Fostino R B Bokosi 1 , Oisin J Shiels 1 , Christopher Richardson 1 , Adam J Trevitt 1 , Sinead T Keaveney 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-01-16 , DOI: 10.1021/acs.joc.3c02545 Fostino R B Bokosi 1 , Oisin J Shiels 1 , Christopher Richardson 1 , Adam J Trevitt 1 , Sinead T Keaveney 1
Affiliation
|
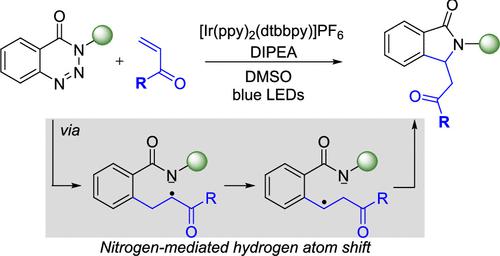
A regioselective visible-light-mediated denitrogenative alkene insertion of 1,2,3-benzotriazin-4(3H)-ones was developed to access 3-substituted isoindolinones, an important structural motif present in many biologically active molecules and natural products. Notably, divergent reactivity was achieved by switching from reported nickel catalysis (where C3-substituted 3,4-dihydroisoquinolin-1(2H)-ones form) to photocatalysis, where photocatalytic denitrogenation and a subsequent nitrogen-mediated hydrogen atom shift lead to exclusive 3-substituted isoindolinone formation. The developed photocatalytic reaction is compatible with activated terminal alkenes and cyclic α,β-unsaturated esters and ketones, with wide functional group tolerance for N-substitution of the 1,2,3-benzotriazin-4(3H)-ones. The utility of this procedure is highlighted by a gram-scale synthesis and postsynthetic amidation. To understand the origin of this unique product selectivity, experimental and computational mechanistic studies were performed.
中文翻译:

1,2,3-苯并三嗪-4(3H)-酮的不同反应性:通过氮介导的氢原子转移实现3-取代异吲哚啉酮的光催化合成
开发了一种区域选择性可见光介导的 1,2,3-苯并三嗪-4(3 H )-酮的脱氮烯烃插入,以获取 3-取代异吲哚啉酮,这是许多生物活性分子和天然产物中存在的重要结构基序。值得注意的是,通过从报道的镍催化(其中形成 C3 取代的 3,4-二氢异喹啉-1(2 H )-酮)转向光催化,实现了不同的反应性,其中光催化脱氮和随后的氮介导的氢原子转移导致排他性3-取代异吲哚啉酮形成。所开发的光催化反应与活化的末端烯烃和环状α,β-不饱和酯和酮相容,对1,2,3-苯并三嗪-4(3 H )-酮的N-取代具有广泛的官能团耐受性。该过程的实用性通过克级合成和合成后酰胺化得以凸显。为了了解这种独特的产物选择性的起源,进行了实验和计算机制研究。
更新日期:2024-01-16
中文翻译:

1,2,3-苯并三嗪-4(3H)-酮的不同反应性:通过氮介导的氢原子转移实现3-取代异吲哚啉酮的光催化合成
开发了一种区域选择性可见光介导的 1,2,3-苯并三嗪-4(3 H )-酮的脱氮烯烃插入,以获取 3-取代异吲哚啉酮,这是许多生物活性分子和天然产物中存在的重要结构基序。值得注意的是,通过从报道的镍催化(其中形成 C3 取代的 3,4-二氢异喹啉-1(2 H )-酮)转向光催化,实现了不同的反应性,其中光催化脱氮和随后的氮介导的氢原子转移导致排他性3-取代异吲哚啉酮形成。所开发的光催化反应与活化的末端烯烃和环状α,β-不饱和酯和酮相容,对1,2,3-苯并三嗪-4(3 H )-酮的N-取代具有广泛的官能团耐受性。该过程的实用性通过克级合成和合成后酰胺化得以凸显。为了了解这种独特的产物选择性的起源,进行了实验和计算机制研究。

































 京公网安备 11010802027423号
京公网安备 11010802027423号