当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio- and Diastereoselective Highly Strained Alkylidenecyclobutane Isomerization/Hydroacylation: Synthesis of Multisubstituted Cyclobutanes with Consecutive Stereocenters
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-01-16 , DOI: 10.1021/acscatal.3c05616 Kai-Qiang Tian 1 , Shi-Jiao Zhang 2 , Jun Zhao 1 , Gui-Yun Duan 1 , Qiao-Ling Wang 3 , Guo-Hao Cui 3 , Rui Guo 3 , Hong-Shuang Li 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-01-16 , DOI: 10.1021/acscatal.3c05616 Kai-Qiang Tian 1 , Shi-Jiao Zhang 2 , Jun Zhao 1 , Gui-Yun Duan 1 , Qiao-Ling Wang 3 , Guo-Hao Cui 3 , Rui Guo 3 , Hong-Shuang Li 1
Affiliation
|
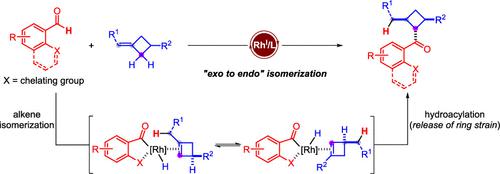
The regio- and diastereoselective alkene isomerization and hydrofunctionalization sequence enabled by transition-metal complexes allows rapid activation and assembly of the C(sp3)–H bond that is either adjacent or distal to the initial double bond, which has been a longstanding challenge in this field. Herein, we develop unusual rhodium-catalyzed isomerization of alkylidenecyclobutanes with subsequent hydroacylation reaction to provide multisubstituted cyclobutanes with continuous stereocenters. Note that this tandem process features a good regio- and diastereoselectivity profile. Isotopic labeling experiments support the “exo to endo” migration of the double bond to a coordinated cyclobutene that is responsible for the deuterium incorporation observed in the cyclobutane product.
中文翻译:

区域和非对映选择性高张力亚烷基环丁烷异构化/加氢酰化:具有连续立构中心的多取代环丁烷的合成
由过渡金属配合物实现的区域和非对映选择性烯烃异构化和氢官能化序列允许快速激活和组装与初始双键相邻或远端的C(sp 3 )–H 键,这一直是化学领域中长期存在的挑战。这个领域。在此,我们开发了亚烷基环丁烷的不寻常的铑催化异构化和随后的加氢酰化反应,以提供具有连续立体中心的多取代环丁烷。请注意,该串联过程具有良好的区域选择性和非对映选择性。同位素标记实验支持双键向配位环丁烯的“外向内”迁移,该环丁烯负责在环丁烷产物中观察到的氘掺入。
更新日期:2024-01-16
中文翻译:

区域和非对映选择性高张力亚烷基环丁烷异构化/加氢酰化:具有连续立构中心的多取代环丁烷的合成
由过渡金属配合物实现的区域和非对映选择性烯烃异构化和氢官能化序列允许快速激活和组装与初始双键相邻或远端的C(sp 3 )–H 键,这一直是化学领域中长期存在的挑战。这个领域。在此,我们开发了亚烷基环丁烷的不寻常的铑催化异构化和随后的加氢酰化反应,以提供具有连续立体中心的多取代环丁烷。请注意,该串联过程具有良好的区域选择性和非对映选择性。同位素标记实验支持双键向配位环丁烯的“外向内”迁移,该环丁烯负责在环丁烷产物中观察到的氘掺入。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号