当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral phosphoric acid-catalyzed enantioselective synthesis of functionalized pyrrolinones containing a geminal diamine core via an aza-Friedel–Crafts reaction of newly developed pyrrolinone ketimines
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-01-15 , DOI: 10.1039/d3qo01909h
Tong Zhang 1 , Zhen-Hua Wang 1 , Yong Li 2 , Jian-Qiang Zhao 1 , Yong You 1 , Yan-Ping Zhang 1 , Jun-Qing Yin 1 , Wei-Cheng Yuan 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-01-15 , DOI: 10.1039/d3qo01909h
Tong Zhang 1 , Zhen-Hua Wang 1 , Yong Li 2 , Jian-Qiang Zhao 1 , Yong You 1 , Yan-Ping Zhang 1 , Jun-Qing Yin 1 , Wei-Cheng Yuan 1
Affiliation
|
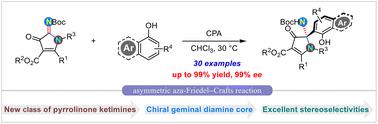
A class of novel pyrrolinone ketimines was used in the catalytic asymmetric aza-Friedel–Crafts reaction with phenolic compounds for the first time. Under the catalysis of a chiral phosphoric acid catalyst, various functionalized pyrrolinone derivatives bearing a geminal diamine core were constructed in good to excellent yields (up to 99%) with high enantioselectivities (up to 99% ee). The scale-up reaction and further transformations of the product also demonstrated the potential application of this protocol.
中文翻译:

通过新开发的吡咯啉酮酮亚胺的氮杂-弗里德尔-克来福特反应,手性磷酸催化对映选择性合成含有偕二胺核心的官能化吡咯啉酮
一类新型吡咯啉酮亚胺首次用于与酚类化合物的催化不对称氮杂弗里德尔-克来福特反应。在手性磷酸催化剂的催化下,以良好至优异的收率(高达99%)和高对映选择性(高达99%ee)构建了各种带有偕二胺核的官能化吡咯啉酮衍生物。产品的放大反应和进一步转化也证明了该方案的潜在应用。
更新日期:2024-01-19
中文翻译:

通过新开发的吡咯啉酮酮亚胺的氮杂-弗里德尔-克来福特反应,手性磷酸催化对映选择性合成含有偕二胺核心的官能化吡咯啉酮
一类新型吡咯啉酮亚胺首次用于与酚类化合物的催化不对称氮杂弗里德尔-克来福特反应。在手性磷酸催化剂的催化下,以良好至优异的收率(高达99%)和高对映选择性(高达99%ee)构建了各种带有偕二胺核的官能化吡咯啉酮衍生物。产品的放大反应和进一步转化也证明了该方案的潜在应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号