背景
乙型肝炎病毒 (HBV) 感染是一个重大的公共卫生问题。HBV 感染后,病毒抗原改变免疫平衡,有利于病毒逃逸。萝卜硫素 (SFN) 是一种传统中药。它调节多种生物活性,包括抗炎、抗癌和抗病毒。然而,以前很少有研究报道 SFN 可以抑制 HBV 感染。
方法
采用免疫活性 HBV CBA/CaJ 小鼠模型和共培养模型探讨 SFN 对 HBV 的影响以及 SFN 是否改变了 HBV 感染后的免疫平衡。
结果
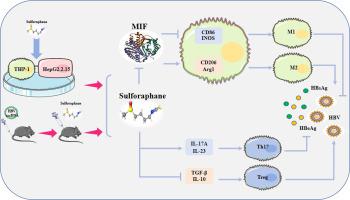
我们发现 SFN 能够降低 HBV 感染小鼠血清和肝组织中的 HBV DNA 、 cccDNA 、 HBsAg 、 HBeAg 和 HBcAg 水平。体外和体内实验表明,SFN 可显著提高 HBV 感染后巨噬细胞上 Cd86 和 iNOS 的表达,抑制 Arg1 的表达。SFN 给药后,肝组织和血清中 Th17 标志物显著升高。外周血中 Treg 细胞比例无显著变化,但 Th17 细胞比例显著增加,Treg/Th17 比值显著降低。使用网络药理学方法,我们预测巨噬细胞迁移抑制因子 (MIF) 是 SFN 的潜在靶点,并进一步验证 HBV 感染后 MIF 表达显着增加,SFN 在体外和体内均显著抑制 MIF 表达。MIF 过表达后 HBV 标志物 (p>0.05) 呈上升趋势。MIF 的过表达与 SFN 的使用相结合导致 HBV 标志物的表达显著逆转和巨噬细胞向 M1 表型的极化。
结论
我们的结果表明,免疫活性 HBV CBA/CaJ 小鼠模型是评估 HBV 感染的良好模型。SFN 可抑制 HBV 标志物的表达,促进 HBV 感染后巨噬细胞向 M1 表型极化,改变 Treg 和 Th17 细胞的比例。我们的研究结果表明,SFN 通过抑制 MIF 的表达和促进巨噬细胞向 M1 表型极化来抑制 HBV 感染,这说明了 HBV 感染的一种有前途的治疗方法。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Sulforaphane effectively inhibits HBV by altering Treg/Th17 immune balance and the MIF-macrophages polarizing axis in vitro and in vivo
Background
Hepatitis B virus (HBV) infection is a major public health problem. After HBV infection, viral antigens shift the immune balance in favor of viral escape. Sulforaphane (SFN) is a traditional Chinese medicine.It regulates multi-biological activities, including anti-inflammation, anticancer, and antiviral. However, few studies reported that SFN can inhibit HBV infection before.
Methods
An immunocompetent HBV CBA/CaJ mouse model and a co-culture model were used to explore the effect of SFN on HBV and whether SFN altered the immune balance after HBV infection.
Results
We found that SFN was able to reduce HBV DNA, cccDNA, HBsAg, HBeAg, and HBcAg levels in serum and liver tissues of HBV-infected mice. In vitro and in vivo experiments showed that SFN could significantly increase the expression of Cd86 and iNOS and inhibit the expression of Arg1 on macrophages after HBV infection. After SFN administration, Th17 markers in liver tissue and serum were significantly increased. There was no significant changes in the proportion of Treg cells in peripheral blood, but a significant increase in the proportion of Th17 cells and decrease of the Treg/Th17 ratio. Using a network pharmacology approach, we predicted macrophage migration inhibitory factor (MIF) as a potential target of SFN and further validated that MIF expression was significantly increased after HBV infection and SFN significantly inhibited MIF expression both in vitro and in vivo. There was an upward trend in HBV markers (p>0.05) after MIF overexpression. Overexpression of MIF combined with the use of SFN resulted in a significant reversion in the expression of HBV markers and polarization of macrophages towards the M1 phenotype.
Conclusion
Our results indicated that immunocompetent HBV CBA/CaJ mouse model is a good model to evaluate HBV infection. SFN could inhibit the expression of HBV markers, promote polarization of macrophages towards the M1 phenotype after HBV infection, change the proportion of Treg and Th17 cells. Our findings demonstrate that SFN inhibit HBV infection by inhibiting the expression of MIF and promoting the polarization of macrophages towards the M1 phenotype, which illustrates a promising therapeutic approach in HBV infection.





















































 京公网安备 11010802027423号
京公网安备 11010802027423号