Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2024-01-13 , DOI: 10.1007/s10593-024-03271-w
Andrey D. Vinokurov , Taygib M. Iliyasov , Kirill A. Karpenko , Radmir N. Akchurin , Yana V. Derkach , Anatoly N. Vereshchagin
|
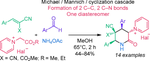
A novel four-component diastereoselective synthesis of piperidin-2-one salts containing a quaternized pyridine unit is reported. The Michael–Mannich cascade was conducted using Michael acceptors, pyridinium ylides, aromatic aldehydes, and ammonium acetate in methanol. It is a convenient approach to the synthesis of 1-((3SR,4RS,6SR)-4,6-diaryl-5,5-dicyano-2-oxopiperidin-3-yl)pyridin-1-ium halides with three stereocenters in 48–84% yield or 1-[(3SR,4RS,5RS,6SR)-4,6-diaryl-5-cyano-5-(methoxycarbonyl)-2-oxopiperidin-3-yl]-pyridin-1-ium halides with four stereocenters in 44–74%. This reaction is highly stereoselective. Only one diastereomer was formed. Ammonium acetate plays a dual role, acting as a base and as a nitrogen source. Structures of the synthesized compounds were confirmed by 1H, 13C NMR, IR, and mass spectra. The formation of a single diastereomer was confirmed by singe crystal X-ray diffraction studies. Products were obtained by simple filtration, and other purification methods as column chromatography were not necessary.
中文翻译:

从吡啶鎓叶立德、醛、迈克尔受体和乙酸铵高度非对映选择性合成吡啶鎓取代的哌啶-2-酮
报道了一种新型四组分非对映选择性合成含有季铵化吡啶单元的哌啶-2-酮盐。迈克尔-曼尼希级联是使用迈克尔受体、吡啶叶立德、芳香醛和乙酸铵在甲醇中进行的。这是合成 1-((3 SR ,4 RS ,6 SR )-4,6-二芳基-5,5-二氰基-2-氧代哌啶-3-基)吡啶-1-鎓卤化物的便捷方法三个立构中心,产率 48–84% 或 1-[(3 SR ,4 RS ,5 RS ,6 SR )-4,6-二芳基-5-氰基-5-(甲氧基羰基)-2-氧代哌啶-3-基] -pyridin-1-ium 卤化物,具有 44-74% 的四个立构中心。该反应具有高度立体选择性。仅形成一种非对映异构体。醋酸铵具有双重作用,既充当碱又充当氮源。合成化合物的结构通过1 H、13 C NMR、IR 和质谱得到证实。通过单晶X射线衍射研究证实了单一非对映异构体的形成。通过简单过滤即可得到产物,不需要柱层析等其他纯化方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号