当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bicyclic Peptide Ligands Pulled out of Cysteine-Rich Peptide Libraries
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-04-17 , DOI: 10.1021/ja400461h Shiyu Chen 1 , Inmaculada Rentero Rebollo 1 , Sergey A. Buth 1 , Julia Morales-Sanfrutos 1 , Jeremy Touati 1 , Petr G. Leiman 1 , Christian Heinis 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-04-17 , DOI: 10.1021/ja400461h Shiyu Chen 1 , Inmaculada Rentero Rebollo 1 , Sergey A. Buth 1 , Julia Morales-Sanfrutos 1 , Jeremy Touati 1 , Petr G. Leiman 1 , Christian Heinis 1
Affiliation
|
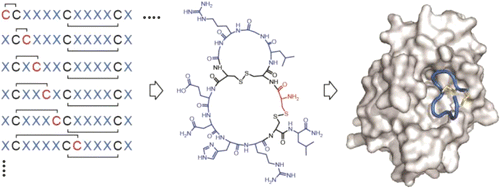
Bicyclic peptide ligands were found to have good binding affinity and target specificity. However, the method applied to generate bicyclic ligands based on phage-peptide alkylation is technically complex and limits its application to specialized laboratories. Herein, we report a method that involves a simpler and more robust procedure that additionally allows screening of structurally more diverse bicyclic peptide libraries. In brief, phage-encoded combinatorial peptide libraries of the format X(m)CX(n)CX(o)CX(p) are oxidized to connect two pairs of cysteines (C). This allows the generation of 3 × (m + n + o + p) different peptide topologies because the fourth cysteine can appear in any of the (m + n + o + p) randomized amino acid positions (X). Panning of such libraries enriched strongly peptides with four cysteines and yielded tight binders to protein targets. X-ray structure analysis revealed an important structural role of the disulfide bridges. In summary, the presented approach offers facile access to bicyclic peptide ligands with good binding affinities.
中文翻译:

从富含半胱氨酸的肽库中提取的双环肽配体
发现双环肽配体具有良好的结合亲和力和靶标特异性。然而,基于噬菌体肽烷基化生成双环配体的方法在技术上很复杂,并限制了其在专业实验室的应用。在此,我们报告了一种方法,该方法涉及更简单、更可靠的程序,该程序还允许筛选结构更多样化的双环肽库。简而言之,X(m)CX(n)CX(o)CX(p) 格式的噬菌体编码组合肽库被氧化以连接两对半胱氨酸 (C)。这允许生成 3 × (m + n + o + p) 不同的肽拓扑结构,因为第四个半胱氨酸可以出现在 (m + n + o + p) 随机氨基酸位置 (X) 中的任何一个。此类文库的淘选丰富了具有四个半胱氨酸的强肽,并产生了与蛋白质目标的紧密结合。X 射线结构分析揭示了二硫键的重要结构作用。总之,所提出的方法提供了容易获得具有良好结合亲和力的双环肽配体。
更新日期:2013-04-17
中文翻译:

从富含半胱氨酸的肽库中提取的双环肽配体
发现双环肽配体具有良好的结合亲和力和靶标特异性。然而,基于噬菌体肽烷基化生成双环配体的方法在技术上很复杂,并限制了其在专业实验室的应用。在此,我们报告了一种方法,该方法涉及更简单、更可靠的程序,该程序还允许筛选结构更多样化的双环肽库。简而言之,X(m)CX(n)CX(o)CX(p) 格式的噬菌体编码组合肽库被氧化以连接两对半胱氨酸 (C)。这允许生成 3 × (m + n + o + p) 不同的肽拓扑结构,因为第四个半胱氨酸可以出现在 (m + n + o + p) 随机氨基酸位置 (X) 中的任何一个。此类文库的淘选丰富了具有四个半胱氨酸的强肽,并产生了与蛋白质目标的紧密结合。X 射线结构分析揭示了二硫键的重要结构作用。总之,所提出的方法提供了容易获得具有良好结合亲和力的双环肽配体。































 京公网安备 11010802027423号
京公网安备 11010802027423号