当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing Coimmobilization Capacity of Schwertmannite for Arsenic and Cadmium through pH Elevation after Chemical Oxidation
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2024-01-11 , DOI: 10.1021/acsestengg.3c00363 Xiaomeng Wang 1 , Lijie Wang 1 , Jingran Fu 1 , Yiming Zhang 1 , Yan Dong 1 , Guanyu Zheng 1 , Lixiang Zhou 1
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2024-01-11 , DOI: 10.1021/acsestengg.3c00363 Xiaomeng Wang 1 , Lijie Wang 1 , Jingran Fu 1 , Yiming Zhang 1 , Yan Dong 1 , Guanyu Zheng 1 , Lixiang Zhou 1
Affiliation
|
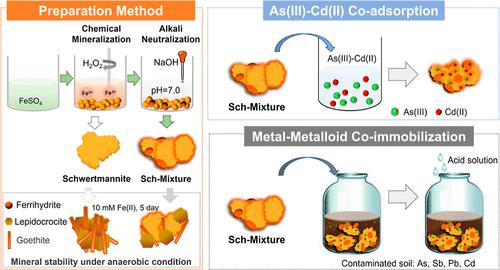
Schwertmannite (Sch) is a promising environmental functional material for remediation of arsenic pollution, particularly arsenite [As(III)], due to its unique adsorption mechanism. However, its application in metal–metalloid cocontamination is greatly limited by its ineffective immobilization of cationic metals. In this study, a preparation method of chemical oxidation–pH elevation was proposed to optimize the immobilization capacity of schwertmannite for As and cadmium [Cd(II)], which referred to promoting solution pH to neutral conditions after FeSO4–H2O2 chemical oxidation. The resultant schwertmannite mixture (Sch-M) had a slightly increased Fe content (by 14.2–17.7%) and a reduced sulfate content (by 24.5–42.1%) compared to Sch. Sch-M displayed stronger mineral phase stability under anaerobic Fe(II)-catalyzed condition. The adsorption capacity of Sch-M for As(III) was basically consistent with that of Sch (172.9–211.3 vs 196.5 mg/g) and reached 19.7–22.4 mg/g for Cd(II) at pH 6.5. Sch-M performed better stabilization effects for metals and metalloids in a contaminated soil than zerovalent iron. In addition, its passivation ability was resistant to acidic conditions (pH 2.6). The practical application of the Sch-based environmental functional material in the remediation of metal(loid)-contaminated soils is worthy of in-depth study.
中文翻译:

通过化学氧化后 pH 值升高增强施威特曼石对砷和镉的共固定化能力
施韦特曼石(Sch)由于其独特的吸附机制,是一种很有前景的砷污染修复环境功能材料,特别是亚砷酸盐[As(III)]。然而,由于其对阳离子金属的固定效果不佳,其在金属-类金属共污染中的应用受到很大限制。本研究提出了一种化学氧化-pH升高的制备方法来优化施威特曼石对As和镉[Cd(II)]的固定能力,即FeSO 4 –H 2 O 2后将溶液pH升至中性条件。化学氧化。与 Sch 相比,所得施威特曼石混合物 (Sch-M) 的 Fe 含量略有增加(增加了 14.2-17.7%),硫酸盐含量降低了(减少了 24.5-42.1%)。 Sch-M在厌氧Fe(II)催化条件下表现出更强的矿物相稳定性。 Sch-M对As(III)的吸附容量与Sch的吸附容量基本一致(172.9–211.3 vs 196.5 mg/g),并且在pH 6.5时对Cd(II)的吸附容量达到19.7–22.4 mg/g。 Sch-M 对受污染土壤中的金属和类金属的稳定作用优于零价铁。此外,其钝化能力能够抵抗酸性条件(pH 2.6)。 Sch基环境功能材料在金属(类)污染土壤修复中的实际应用值得深入研究。
更新日期:2024-01-11
中文翻译:

通过化学氧化后 pH 值升高增强施威特曼石对砷和镉的共固定化能力
施韦特曼石(Sch)由于其独特的吸附机制,是一种很有前景的砷污染修复环境功能材料,特别是亚砷酸盐[As(III)]。然而,由于其对阳离子金属的固定效果不佳,其在金属-类金属共污染中的应用受到很大限制。本研究提出了一种化学氧化-pH升高的制备方法来优化施威特曼石对As和镉[Cd(II)]的固定能力,即FeSO 4 –H 2 O 2后将溶液pH升至中性条件。化学氧化。与 Sch 相比,所得施威特曼石混合物 (Sch-M) 的 Fe 含量略有增加(增加了 14.2-17.7%),硫酸盐含量降低了(减少了 24.5-42.1%)。 Sch-M在厌氧Fe(II)催化条件下表现出更强的矿物相稳定性。 Sch-M对As(III)的吸附容量与Sch的吸附容量基本一致(172.9–211.3 vs 196.5 mg/g),并且在pH 6.5时对Cd(II)的吸附容量达到19.7–22.4 mg/g。 Sch-M 对受污染土壤中的金属和类金属的稳定作用优于零价铁。此外,其钝化能力能够抵抗酸性条件(pH 2.6)。 Sch基环境功能材料在金属(类)污染土壤修复中的实际应用值得深入研究。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号