当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tailoring local environment of oxygen vacancies by molybdenum doping on MnO2 for enhanced catalytic oxidation of VOCs
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-01-12 , DOI: 10.1016/j.cej.2024.148703
Yarui Qiao , Shu Yang , Xiaoxia Sun , Cao Liu , Zhilou Liu , Xinxin Li , Lingling Li , Hui Liu , Lei Wang
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-01-12 , DOI: 10.1016/j.cej.2024.148703
Yarui Qiao , Shu Yang , Xiaoxia Sun , Cao Liu , Zhilou Liu , Xinxin Li , Lingling Li , Hui Liu , Lei Wang
|
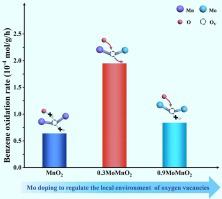
The microenvironment of oxygen vacancies plays an important role in metal oxide catalysts for VOCs catalytic oxidation. A simple strategy was provided to tailor the local environment of oxygen vacancies over MnO2 via Mo doping engineering to enhance its catalytic activity. The 0.3MoMnO2 with Mo/Mn quality ratio of 0.3 showed superior catalytic oxidation performance of benzene and anti-toxicity (H2 O, SO2, and particulate matter). Its benzene oxidation rate at 190℃ was 1.95 × 10−4 mol/g/h, which was about three and two times higher than that of MnO2 and 0.9MoMnO2 , respectively. The Mn-Ov-Mo sites were experimentally verified as the dominant active centers on 0.3MoMnO2 . Through DFT calculation, Mo doping could enhance the oxygen adsorption on oxygen vacancies of MnO2 . The remarkably well activity of Mn-Ov-Mo was rationally explained by the moderate oxygen adsorption energy to maintain a dynamic equilibrium of oxygen adsorption and desorption, which enhanced the oxygen activation, thereby promoting the benzene catalytic oxidation. However, excessive Mo doping induced the formation of Mo-Ov-Mo, and its side-on oxygen adsorption configuration was not conducive to oxygen transfer. This work broadens the understanding of the relationship between the local environment of oxygen vacancies and catalytic activities, which is beneficial for the rational design of efficient VOCs catalysts.
中文翻译:

通过在 MnO2 上掺杂钼来调整氧空位的局部环境,以增强 VOC 的催化氧化
氧空位的微环境在金属氧化物催化剂催化氧化VOCs中起着重要作用。提出了一种简单的策略,通过 Mo 掺杂工程来调整 MnO2 上的局部氧空位环境,以增强其催化活性。 Mo/Mn质量比为0.3的0.3MoMnO2表现出优异的苯催化氧化性能和抗毒性(H2O、SO2和颗粒物)。其在190℃下的苯氧化速率为1.95×10−4 mol/g/h,分别比MnO2和0.9MoMnO2高约3倍和2倍。实验证实 Mn-Ov-Mo 位点是 0.3MoMnO2 上的主要活性中心。通过DFT计算,Mo掺杂可以增强MnO2氧空位上的氧吸附。 Mn-Ov-Mo的良好活性合理地解释为适度的氧吸附能,维持氧吸附和解吸的动态平衡,增强了氧的活化,从而促进苯催化氧化。然而,过量的Mo掺杂会导致Mo-Ov-Mo的形成,其侧向氧吸附构型不利于氧转移。这项工作拓宽了对氧空位局部环境与催化活性之间关系的理解,有利于高效VOCs催化剂的合理设计。
更新日期:2024-01-12
中文翻译:

通过在 MnO2 上掺杂钼来调整氧空位的局部环境,以增强 VOC 的催化氧化
氧空位的微环境在金属氧化物催化剂催化氧化VOCs中起着重要作用。提出了一种简单的策略,通过 Mo 掺杂工程来调整 MnO2 上的局部氧空位环境,以增强其催化活性。 Mo/Mn质量比为0.3的0.3MoMnO2表现出优异的苯催化氧化性能和抗毒性(H2O、SO2和颗粒物)。其在190℃下的苯氧化速率为1.95×10−4 mol/g/h,分别比MnO2和0.9MoMnO2高约3倍和2倍。实验证实 Mn-Ov-Mo 位点是 0.3MoMnO2 上的主要活性中心。通过DFT计算,Mo掺杂可以增强MnO2氧空位上的氧吸附。 Mn-Ov-Mo的良好活性合理地解释为适度的氧吸附能,维持氧吸附和解吸的动态平衡,增强了氧的活化,从而促进苯催化氧化。然而,过量的Mo掺杂会导致Mo-Ov-Mo的形成,其侧向氧吸附构型不利于氧转移。这项工作拓宽了对氧空位局部环境与催化活性之间关系的理解,有利于高效VOCs催化剂的合理设计。

































 京公网安备 11010802027423号
京公网安备 11010802027423号