当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visualization of the Active Sites of Zinc–Chromium Oxides and the CO/H2 Activation Mechanism in Direct Syngas Conversion
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-11 , DOI: 10.1021/jacs.3c07332 Yuxiang Chen 1, 2 , Shaobo Han 1 , Xiulian Pan 1 , Feng Jiao 1 , Wei Liu 1 , Yang Pan 3 , Xinhe Bao 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-11 , DOI: 10.1021/jacs.3c07332 Yuxiang Chen 1, 2 , Shaobo Han 1 , Xiulian Pan 1 , Feng Jiao 1 , Wei Liu 1 , Yang Pan 3 , Xinhe Bao 1
Affiliation
|
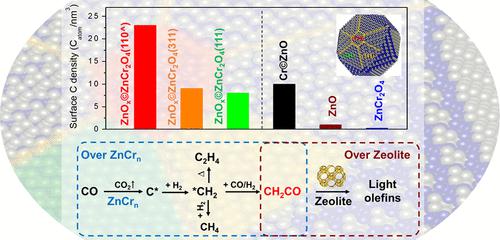
Despite wide studies demonstrating the versatility of the metal oxide-zeolite (OXZEO) catalyst concept to tackle the selectivity challenge in syngas chemistry, the active sites of metal oxides and the mechanism of CO/H2 activation remain to be elucidated. Herein, we demonstrate experimentally the role of Cr in zinc–chromium oxides and unveil visually, for the first time, the active sites for CO activation employing scanning transmission electron microscopy-electron energy loss spectroscopy using the volumetric density of surface carbon species as a descriptor. The ZnCr2O4 spinel surface with atomic ZnOx overlayer is the most active site for C–O bond dissociation, particularly at the narrow ZnCr2O4(110) facets constrained between the (311) and (111) facets, followed by the Cr-doped wurtzite ZnO surface. In comparison, the surfaces of ZnCr2O4 with aggregated ZnOx overlayers, pure ZnO, and the stoichiometric ZnCr2O4 exhibit a significantly lower activity. In situ synchrotron-based vacuum ultraviolet photoionization mass spectrometric study on different temperature programmed surface reactions with isotopes of C18O, 13CO, and D2 validates direct CO dissociation over ZnCrn oxides in CO, forming CH2 and further to hydrocarbons if H2 is present and CH2CO intermediates in syngas. The activity of CO dissociation and hydrogenation over ZnCrn oxides correlates well with the syngas-to-light-olefins activity of ZnCrn-SAPO-18 composite catalysts as a function of the Cr/Zn ratio.
中文翻译:

直接合成气转化中锌铬氧化物活性位点和 CO/H2 活化机制的可视化
尽管广泛的研究表明金属氧化物-沸石(OXZEO)催化剂概念在解决合成气化学中的选择性挑战方面具有多功能性,但金属氧化物的活性位点和CO/H 2活化机制仍有待阐明。在此,我们通过实验证明了铬在锌铬氧化物中的作用,并首次采用扫描透射电子显微镜-电子能量损失光谱法,使用表面碳物种的体积密度作为描述符,直观地揭示了CO活化的活性位点。具有原子 ZnO x覆盖层的 ZnCr 2 O 4尖晶石表面是 C-O 键解离最活跃的位点,特别是在 (311) 和 (111) 面之间限制的狭窄 ZnCr 2 O 4 (110) 面,其次是Cr掺杂纤锌矿ZnO表面。相比之下,具有聚集的ZnO x覆盖层的ZnCr 2 O 4 、纯ZnO和化学计量的ZnCr 2 O 4的表面表现出显着较低的活性。基于原位同步加速器的真空紫外光电离质谱研究,对 C 18 O、 13 CO 和 D 2同位素的不同程序升温表面反应验证了 CO 中 ZnCr n氧化物的直接 CO 离解,形成 CH 2 ,如果 H 则进一步形成碳氢化合物合成气中存在2和CH 2 CO中间体。 ZnCr n氧化物上的 CO 解离和加氢活性与 ZnCr n -SAPO-18 复合催化剂的合成气制轻质烯烃活性密切相关,作为 Cr/Zn 比的函数。
更新日期:2024-01-11
中文翻译:

直接合成气转化中锌铬氧化物活性位点和 CO/H2 活化机制的可视化
尽管广泛的研究表明金属氧化物-沸石(OXZEO)催化剂概念在解决合成气化学中的选择性挑战方面具有多功能性,但金属氧化物的活性位点和CO/H 2活化机制仍有待阐明。在此,我们通过实验证明了铬在锌铬氧化物中的作用,并首次采用扫描透射电子显微镜-电子能量损失光谱法,使用表面碳物种的体积密度作为描述符,直观地揭示了CO活化的活性位点。具有原子 ZnO x覆盖层的 ZnCr 2 O 4尖晶石表面是 C-O 键解离最活跃的位点,特别是在 (311) 和 (111) 面之间限制的狭窄 ZnCr 2 O 4 (110) 面,其次是Cr掺杂纤锌矿ZnO表面。相比之下,具有聚集的ZnO x覆盖层的ZnCr 2 O 4 、纯ZnO和化学计量的ZnCr 2 O 4的表面表现出显着较低的活性。基于原位同步加速器的真空紫外光电离质谱研究,对 C 18 O、 13 CO 和 D 2同位素的不同程序升温表面反应验证了 CO 中 ZnCr n氧化物的直接 CO 离解,形成 CH 2 ,如果 H 则进一步形成碳氢化合物合成气中存在2和CH 2 CO中间体。 ZnCr n氧化物上的 CO 解离和加氢活性与 ZnCr n -SAPO-18 复合催化剂的合成气制轻质烯烃活性密切相关,作为 Cr/Zn 比的函数。






























 京公网安备 11010802027423号
京公网安备 11010802027423号