Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-01-09 , DOI: 10.1002/adsc.202301303
Rosa Pich 1 , Sonia Ladeira 2 , Pierre Lavedan 2 , Jean-François Lahitte 3 , Jean-Christophe Remigy 3 , Daniel Pla 4 , Montserrat Gómez 5
|
Introduction
The efficiency and selectivity of multicomponent reactions in which three or more substrates are streamlined towards the formation of a single product enable the development of novel synthetic strategies.1 In particular, the Cu(I)-catalyzed four-component coupling of amines, aldehydes, alkynes and CO2 is a versatile and attractive method for the synthesis of oxazolidinones, a class of heterocyclic scaffold exhibiting applications in medicinal chemistry (novel antibiotics against microbial resistance, antitubercular activity, antiviral and anti-cancer agents via topoisomerase II inhibition),2 molecular chemistry (chiral auxiliaries in enantioselective catalysis)3 and materials science (polymeric biocidal films, flame retardants),4 due to their unique structural and hydrogen bonding properties.
The field of metal-catalyzed four-component coupling reactions towards oxazolidinone synthesis is rapidly evolving,5 but most of the catalytic systems are based on Cu(I)6 (few contributions involve Cu(II) catalytic precursors7) or combinations thereof with engineered solvents such as ionic liquids to enhance the recyclability of the resulting catalytic systems.8
From a mechanistic point of view, the seminal work reported by Li and coworkers proposed a mechanism for the A3-coupling carboxylative cyclization through the formation of the corresponding propargylamine intermediate and also pointing to a synergistic effect between the amine and CO2.9 Later on, Hu and coworkers studied this four-component reaction using the preformed carbamate (coming from the reaction between the primary amine and CO2) as reactant, proving that these reaction conditions permitted the synthesis of oxazolidinones using a lower catalyst loading (5 mol% of CuI);10 these authors also studied the immobilization of Cu(II) salts on nano-Fe3O4 for recycling purposes.11 More recently, Chen's group studied this type of reaction in neat water and in the presence of molecular sieves which act as promoter for CO2 absorption/fixation. Authors used diazabicyclo[5.4.0]undec-7-ene (DBU) as co-catalyst forming the corresponding carbamate intermediate, which allows the CO2 moiety transfer to the corresponding primary amine.12
To the best of our knowledge, mechanistic studies permitting to identify both organic compounds and Cu-based coordination complexes as intermediates of the four-component reaction, have not previously been reported.
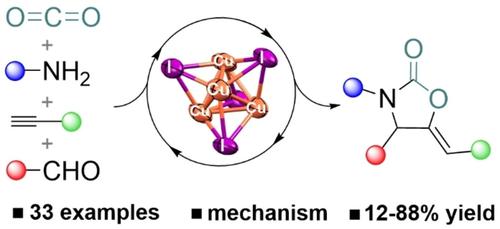
In this frame, herein we report a Cu(I)-catalyzed tandem four-component carboxylative coupling of amines, aldehydes and terminal alkynes under CO2 pressure in ethanol, offering a green and non-hazardous alternative to classical metal-catalyzed protocols employing isocyanates.13 The unrivaled results obtained with CuI catalytic systems prompted us to study the mechanistic insights of this transformation with the aim of gaining a better insight on the active catalytic species and key intermediates involved.
中文翻译:

CuI 催化四组分反应合成恶唑烷酮的机理见解
介绍
多组分反应的效率和选择性,其中三种或多种底物被简化以形成单一产物,使得新型合成策略的开发成为可能。1特别是,Cu(I) 催化的胺、醛、炔和 CO 2的四组分偶联是合成恶唑烷酮的一种通用且有吸引力的方法,恶唑烷酮是一类杂环支架,在药物化学(针对抗生素的新型抗生素)中展示了应用。微生物耐药性、抗结核活性、通过拓扑异构酶 II 抑制的抗病毒和抗癌药物)、2分子化学(对映选择性催化中的手性助剂)3和材料科学(聚合物杀菌膜、阻燃剂)4由于其独特的结构和氢键特性。
恶唑烷酮合成的金属催化四组分偶联反应领域正在迅速发展,5但大多数催化系统都是基于 Cu(I) 6(很少涉及 Cu(II) 催化前体7)或其组合与工程化的溶剂如离子液体,以增强所得催化系统的可回收性。8
从机理的角度来看,Li及其同事报道的开创性工作提出了一种通过形成相应的炔丙胺中间体进行A 3偶联羧基环化的机制,并指出了胺和CO 2之间的协同效应。9后来,Hu 和同事研究了这种四组分反应,使用预制的氨基甲酸酯(来自伯胺和 CO 2之间的反应)作为反应物,证明这些反应条件允许使用较低的催化剂负载量合成恶唑烷酮(5 CuI 的 mol%); 10这些作者还研究了将 Cu(II) 盐固定在纳米 Fe 3 O 4上以用于回收目的。11最近,Chen 的研究小组在纯水中和分子筛存在下研究了此类反应,分子筛充当 CO 2吸收/固定的促进剂。作者使用二氮杂双环[5.4.0]十一碳7-烯(DBU)作为助催化剂,形成相应的氨基甲酸酯中间体,使CO 2部分转移到相应的伯胺上。12
据我们所知,之前尚未报道过能够将有机化合物和铜基配位配合物鉴定为四组分反应中间体的机理研究。
在此框架中,我们报告了在乙醇中CO 2压力下,Cu(I)催化的胺、醛和末端炔烃的串联四组分羧化偶联,为使用异氰酸酯的传统金属催化方案提供了一种绿色且无害的替代方案。13 CuI 催化系统获得的无与伦比的结果促使我们研究这种转化的机制,目的是更好地了解所涉及的活性催化物质和关键中间体。































 京公网安备 11010802027423号
京公网安备 11010802027423号