当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyrimido[5,4-c]quinolines: Synthesis from 3,4-Di-functionallized Quinoline, Reactivity and Biological Activities
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2024-01-09 , DOI: 10.1002/cbdv.202301968 Moustafa A Gouda 1, 2 , Ameen A Abu-Hashem 3, 4 , Tahah A Ameen 3 , Saif H Althagafi 5 , Wafaa S Hamama 2 , Abdel-Galil M Khalil 2
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2024-01-09 , DOI: 10.1002/cbdv.202301968 Moustafa A Gouda 1, 2 , Ameen A Abu-Hashem 3, 4 , Tahah A Ameen 3 , Saif H Althagafi 5 , Wafaa S Hamama 2 , Abdel-Galil M Khalil 2
Affiliation
|
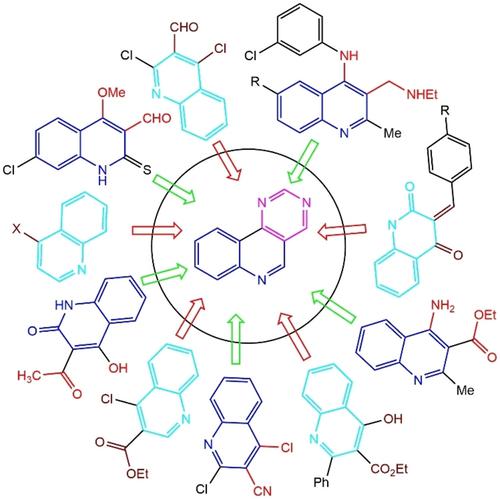
Quinoline and pyrimidine moieties are ubiquitous components in both natural and synthetic compounds, showcasing diverse applications. The fusion of these well-known structures into hybrid molecules has garnered attention due to their intriguing biological properties. Particularly in the field of medicinal chemistry, numerous studies in the last decade have focused on pyrimido[5,4-c]quinoline ring systems (PyQs5,4-c). This review elucidates the synthesis of PyQs5,4-c and their derivatives using 3,4-difunctionalized quinoline as a key starting material. The preparation of PyQs5,4-c involves a series of chemical transformations, including the Friedländer, Ullmann and Biginelli reaction, Vilsmeier-Haack formylation, Suzuki coupling, and a one-pot three-component reaction. These synthetic routes not only offer access to diverse PyQs5,4-c derivatives
中文翻译:

嘧啶并[5,4-c]喹啉:3,4-双官能化喹啉的合成、反应性和生物活性
喹啉和嘧啶部分是天然和合成化合物中普遍存在的成分,展示了多种应用。将这些众所周知的结构融合成杂化分子因其有趣的生物学特性而引起了人们的关注。特别是在药物化学领域,过去十年的大量研究都集中在嘧啶并[5,4- c ]喹啉环系统(PyQs5,4-c)。本综述阐明了使用 3,4-双官能化喹啉作为关键起始原料合成 PyQs5,4-c 及其衍生物。 PyQs5,4-c的制备涉及一系列化学转化,包括Friedländer 、Ullmann和Biginelli反应、Vilsmeier-Haack甲酰化、Suzuki偶联和一锅三组分反应。这些合成路线不仅提供了多种 PyQs5,4-c 衍生物的获得
更新日期:2024-01-09
中文翻译:

嘧啶并[5,4-c]喹啉:3,4-双官能化喹啉的合成、反应性和生物活性
喹啉和嘧啶部分是天然和合成化合物中普遍存在的成分,展示了多种应用。将这些众所周知的结构融合成杂化分子因其有趣的生物学特性而引起了人们的关注。特别是在药物化学领域,过去十年的大量研究都集中在嘧啶并[5,4- c ]喹啉环系统(PyQs5,4-c)。本综述阐明了使用 3,4-双官能化喹啉作为关键起始原料合成 PyQs5,4-c 及其衍生物。 PyQs5,4-c的制备涉及一系列化学转化,包括Friedländer 、Ullmann和Biginelli反应、Vilsmeier-Haack甲酰化、Suzuki偶联和一锅三组分反应。这些合成路线不仅提供了多种 PyQs5,4-c 衍生物的获得






























 京公网安备 11010802027423号
京公网安备 11010802027423号