当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel Benzotriazole-β-lactam Derivatives as Antimalarial Agents: Design, Synthesis, Biological Evaluation and Molecular Docking Studies
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2024-01-08 , DOI: 10.1002/cbdv.202301745 Malihe Aye 1, 2 , Aliasghar Jarrahpour 1 , Zahra Haghighijoo 3 , Roghayeh Heiran 4 , Roya Pournejati 5 , Hamid Reza Karbalaei-Heidari 5 , Veronique Sinou 6 , Jean Michel Brunel 6 , Mehmet Akkurt 7 , Namık Özdemir 8 , Edward Turos 9
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2024-01-08 , DOI: 10.1002/cbdv.202301745 Malihe Aye 1, 2 , Aliasghar Jarrahpour 1 , Zahra Haghighijoo 3 , Roghayeh Heiran 4 , Roya Pournejati 5 , Hamid Reza Karbalaei-Heidari 5 , Veronique Sinou 6 , Jean Michel Brunel 6 , Mehmet Akkurt 7 , Namık Özdemir 8 , Edward Turos 9
Affiliation
|
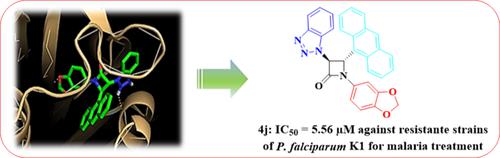
Many people around the world suffer from malaria, especially in tropical or subtropical regions. While malaria medications have shown success in treating malaria, there is still a problem with resistance to these drugs. Herein, we designed and synthesized some structurally novel benzotriazole-β-lactams using 2-(1H-benzo[d][1,2,3]triazol-1-yl)acetic acid as a key intermediate. To synthesize the target molecules, the ketene-imine cycloaddition reaction was employed. First, The reaction of 1H-benzo[d][1,2,3]triazole with 2-bromoacetic acid in aqueous sodium hydroxide yielded 2-(1H-benzo[d][1,2,3]triazol-1-yl)acetic acid. Then, the treatment of 2-(1H-benzo[d][1,2,3]triazol-1-yl)acetic acid with tosyl chloride, triethyl amine, and Schiff base provided new β-lactams in good to moderate yields.The formation of all cycloadducts was confirmed by elemental analysis, FT-IR, NMR and mass spectral data. Moreover, X-ray crystallography was used to determine the relative stereochemistry of 4a compound. The in vitro antimalarial activity test was conducted for each compound against P. falciparum K1. The IC50 values ranged from 5.56 to 25.65 μM. A cytotoxicity profile of the compounds at 200 μM final concentration revealed suitable selectivity of the compounds for malaria treatment. Furthermore, the docking study was carried out for each compound into the P. falciparum dihydrofolate reductase enzyme (PfDHFR) binding site to analyze their possible binding orientation in the active site.
中文翻译:

新型苯并三唑-β-内酰胺衍生物作为抗疟药:设计、合成、生物学评价和分子对接研究
世界各地有许多人患有疟疾,特别是在热带或亚热带地区。虽然疟疾药物在治疗疟疾方面取得了成功,但仍然存在对这些药物产生耐药性的问题。在此,我们以2-(1 H-苯并[ d ][1,2,3]三唑-1-基)乙酸为关键中间体,设计并合成了一些结构新颖的苯并三唑-β-内酰胺。为了合成目标分子,采用了乙烯酮-亚胺环加成反应。首先,1 H-苯并[ d ][1,2,3]三唑与2-溴乙酸在氢氧化钠水溶液中反应,得到2-( 1H-苯并[ d ][1,2,3]三唑-1 -基)乙酸。然后,用甲苯磺酰氯、三乙胺和席夫碱处理 2-(1 H-苯并[ d ][1,2,3]三唑-1-基)乙酸,以良好到中等的收率提供了新的β-内酰胺所有环加合物的形成均通过元素分析、FT-IR、NMR 和质谱数据证实。此外,X射线晶体学用于确定4a化合物的相对立体化学。对每种化合物进行了针对恶性疟原虫K1 的体外抗疟活性测试。 IC 50值范围为 5.56 至 25.65 μM。终浓度为 200 μM 的化合物的细胞毒性特征表明,这些化合物对于疟疾治疗具有合适的选择性。此外,对每种化合物进行了恶性疟原虫二氢叶酸还原酶(PfDHFR)结合位点的对接研究,以分析它们在活性位点中可能的结合方向。
更新日期:2024-01-08
中文翻译:

新型苯并三唑-β-内酰胺衍生物作为抗疟药:设计、合成、生物学评价和分子对接研究
世界各地有许多人患有疟疾,特别是在热带或亚热带地区。虽然疟疾药物在治疗疟疾方面取得了成功,但仍然存在对这些药物产生耐药性的问题。在此,我们以2-(1 H-苯并[ d ][1,2,3]三唑-1-基)乙酸为关键中间体,设计并合成了一些结构新颖的苯并三唑-β-内酰胺。为了合成目标分子,采用了乙烯酮-亚胺环加成反应。首先,1 H-苯并[ d ][1,2,3]三唑与2-溴乙酸在氢氧化钠水溶液中反应,得到2-( 1H-苯并[ d ][1,2,3]三唑-1 -基)乙酸。然后,用甲苯磺酰氯、三乙胺和席夫碱处理 2-(1 H-苯并[ d ][1,2,3]三唑-1-基)乙酸,以良好到中等的收率提供了新的β-内酰胺所有环加合物的形成均通过元素分析、FT-IR、NMR 和质谱数据证实。此外,X射线晶体学用于确定4a化合物的相对立体化学。对每种化合物进行了针对恶性疟原虫K1 的体外抗疟活性测试。 IC 50值范围为 5.56 至 25.65 μM。终浓度为 200 μM 的化合物的细胞毒性特征表明,这些化合物对于疟疾治疗具有合适的选择性。此外,对每种化合物进行了恶性疟原虫二氢叶酸还原酶(PfDHFR)结合位点的对接研究,以分析它们在活性位点中可能的结合方向。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号