当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Imidazodiazepine Analogue, 5-(8-Bromo-6-(pyridin-2-yl)-4H-benzo[f]imidazo[1,5-a][1,4]diazepin-3-yl)oxazole, Provides a Simplified Synthetic Scheme, High Oral Plasma and Brain Exposures, and Produces Antiseizure Efficacy in Mice, and Antiepileptogenic Activity in Neural Networks in Brain Slices from a Patient with Mesial Temporal Lobe Epilepsy
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2024-01-04 , DOI: 10.1021/acschemneuro.3c00555 Dishary Sharmin 1 , Branka Divović 2 , Xingjie Ping 3 , Rok Cerne 3, 4, 5, 6 , Jodi L Smith 4 , Sepideh Rezvanian 1 , Prithu Mondal 1 , Michelle Jean Meyer 1 , Molly E Kiley 1 , Leggy A Arnold 1 , Md Yeunus Mian 1 , Kamal P Pandey 1 , Xiaoming Jin 3 , Jelena R Mitrović 7 , Djordje Djorović 8 , Arnold Lippa 5 , James M Cook 1, 5 , Lalit K Golani 9 , Petra Scholze 10 , Miroslav M Savić 2 , Jeffrey M Witkin 1, 4, 5
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2024-01-04 , DOI: 10.1021/acschemneuro.3c00555 Dishary Sharmin 1 , Branka Divović 2 , Xingjie Ping 3 , Rok Cerne 3, 4, 5, 6 , Jodi L Smith 4 , Sepideh Rezvanian 1 , Prithu Mondal 1 , Michelle Jean Meyer 1 , Molly E Kiley 1 , Leggy A Arnold 1 , Md Yeunus Mian 1 , Kamal P Pandey 1 , Xiaoming Jin 3 , Jelena R Mitrović 7 , Djordje Djorović 8 , Arnold Lippa 5 , James M Cook 1, 5 , Lalit K Golani 9 , Petra Scholze 10 , Miroslav M Savić 2 , Jeffrey M Witkin 1, 4, 5
Affiliation
|
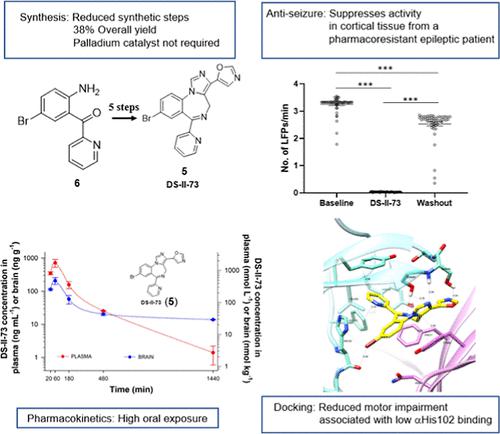
KRM-II-81 (1) is an imidazodiazepine GABAA receptor (GABAAR) potentiator with broad antiseizure efficacy and a low sedative burden. A brominated analogue, DS-II-73 (5), was synthesized and pharmacologically characterized as a potential backup compound as KRM-II-81 moves forward into development. The synthesis from 2-amino-5-bromophenyl)(pyridin-2yl)methanone (6) was processed in five steps with an overall yield of 38% and without the need for a palladium catalyst. GABAAR binding occurred with a Ki of 150 nM, and only 3 of 41 screened binding sites produced inhibition ≥50% at 10 μM, and the potency to induce cytotoxicity was ≥240 mM. DS-II-73 was selective for α2/3/5- over that of α1-containing GABAARs. Oral exposure of plasma and brain of rats was more than sufficient to functionally impact GABAARs. Tonic convulsions in mice and lethality induced by pentylenetetrazol were suppressed by DS-II-73 after oral administration and latencies to clonic and tonic seizures were prolonged. Cortical slice preparations from a patient with pharmacoresistant epilepsy (mesial temporal lobe) showed decreases in the frequency of local field potentials by DS-II-73. As with KRM-II-81, the motor-impairing effects of DS-II-73 were low compared to diazepam. Molecular docking studies of DS-II-73 with the α1β3γ2L-configured GABAAR showed low interaction with α1His102 that is suggested as a potential molecular mechanism for its low sedative side effects. These findings support the viability of DS-II-73 as a backup molecule for its ethynyl analogue, KRM-II-81, with the human tissue data providing translational credibility.
中文翻译:

新的咪唑并二氮杂卓类似物,5-(8-Bromo-6-(pyridin-2-yl)-4H-苯并[f]咪唑并[1,5-a][1,4]二氮杂卓-3-基)恶唑,提供简化的合成方案、高口服血浆和脑暴露,在小鼠中产生抗癫痫功效,以及内侧颞叶癫痫患者脑切片中神经网络的抗癫痫活性
KRM-II-81 (1) 是一种咪唑并二氮杂卓 GABA A受体 (GABAAR) 增强剂,具有广泛的抗癫痫功效和低镇静负担。合成了溴化类似物 DS-II-73 (5),并在药理学上表征为 KRM-II-81 开发过程中的潜在备用化合物。由2-氨基-5-溴苯基)(吡啶-2基)甲酮(6)的合成分五步进行,总收率为38%,并且不需要钯催化剂。 GABAAR 结合的K i为 150 nM,41 个筛选的结合位点中只有 3 个在 10 μM 时产生≥50% 的抑制,诱导细胞毒性的效力≥240 mM。 DS-II-73 对 α2/3/5- 的选择性高于对含有 α1 的 GABAAR。大鼠血浆和大脑的口服暴露足以影响 GABAAR 的功能。口服DS-II-73后,小鼠的强直惊厥和戊四唑引起的致死被抑制,并且阵挛和强直发作的潜伏期延长。 DS-II-73 发现耐药性癫痫患者(内侧颞叶)的皮质切片制剂显示局部场电位频率降低。与 KRM-II-81 一样,DS-II-73 的运动损伤作用比地西泮低。 DS-II-73 与 α1β3γ2L 配置的 GABAAR 的分子对接研究表明,与 α1His102 的相互作用较低,这被认为是其低镇静副作用的潜在分子机制。这些发现支持 DS-II-73 作为其乙炔基类似物 KRM-II-81 的后备分子的可行性,并且人体组织数据提供了翻译可信度。
更新日期:2024-01-04
中文翻译:

新的咪唑并二氮杂卓类似物,5-(8-Bromo-6-(pyridin-2-yl)-4H-苯并[f]咪唑并[1,5-a][1,4]二氮杂卓-3-基)恶唑,提供简化的合成方案、高口服血浆和脑暴露,在小鼠中产生抗癫痫功效,以及内侧颞叶癫痫患者脑切片中神经网络的抗癫痫活性
KRM-II-81 (1) 是一种咪唑并二氮杂卓 GABA A受体 (GABAAR) 增强剂,具有广泛的抗癫痫功效和低镇静负担。合成了溴化类似物 DS-II-73 (5),并在药理学上表征为 KRM-II-81 开发过程中的潜在备用化合物。由2-氨基-5-溴苯基)(吡啶-2基)甲酮(6)的合成分五步进行,总收率为38%,并且不需要钯催化剂。 GABAAR 结合的K i为 150 nM,41 个筛选的结合位点中只有 3 个在 10 μM 时产生≥50% 的抑制,诱导细胞毒性的效力≥240 mM。 DS-II-73 对 α2/3/5- 的选择性高于对含有 α1 的 GABAAR。大鼠血浆和大脑的口服暴露足以影响 GABAAR 的功能。口服DS-II-73后,小鼠的强直惊厥和戊四唑引起的致死被抑制,并且阵挛和强直发作的潜伏期延长。 DS-II-73 发现耐药性癫痫患者(内侧颞叶)的皮质切片制剂显示局部场电位频率降低。与 KRM-II-81 一样,DS-II-73 的运动损伤作用比地西泮低。 DS-II-73 与 α1β3γ2L 配置的 GABAAR 的分子对接研究表明,与 α1His102 的相互作用较低,这被认为是其低镇静副作用的潜在分子机制。这些发现支持 DS-II-73 作为其乙炔基类似物 KRM-II-81 的后备分子的可行性,并且人体组织数据提供了翻译可信度。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号