Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2024-01-10 , DOI: 10.1016/j.abb.2024.109882 Julia Tutzauer 1 , D Stephen Serafin 2 , Tobias Schmidt 3 , Björn Olde 4 , Kathleen M Caron 2 , L M Fredrik Leeb-Lundberg 1
|
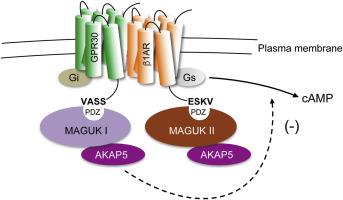
G protein-coupled receptor 30 (GPR30), also named G protein-coupled estrogen receptor (GPER), and the β1-adrenergic receptor (β1AR) are G protein-coupled receptors (GPCR) that are implicated in breast cancer progression. Both receptors contain PSD-95/Discs-large/ZO-1 homology (PDZ) motifs in their C-terminal tails through which they interact in the plasma membrane with membrane-associated guanylate kinase (MAGUK) scaffold proteins, and in turn protein kinase A anchoring protein (AKAP) 5. GPR30 constitutively and PDZ-dependently inhibits β1AR-mediated cAMP production. We hypothesized that this inhibition is a consequence of a plasma membrane complex of these receptors. Using co-immunoprecipitation, confocal immunofluorescence microscopy, and bioluminescence resonance energy transfer (BRET), we show that GPR30 and β1AR reside in close proximity in a plasma membrane complex when transiently expressed in HEK293. Deleting the GPR30 C-terminal PDZ motif (-SSAV) does not interfere with the receptor complex, indicating that the complex is not PDZ-dependent. MCF7 breast cancer cells express GPR30, β1AR, MAGUKs, and AKAP5 in the plasma membrane, and co-immunoprecipitation revealed that these proteins exist in close proximity also under native conditions. Furthermore, expression of GPR30 in MCF7 cells constitutively and PDZ-dependently inhibits β1AR-mediated cAMP production. AKAP5 also inhibits β1AR-mediated cAMP production, which is not additive with GPR30-promoted inhibition. These results argue that GPR30 and β1AR form a PDZ-independent complex in MCF7 cells through which GPR30 constitutively and PDZ-dependently inhibits β1AR signaling via receptor interaction with MAGUKs and AKAP5.
中文翻译:

G 蛋白偶联雌激素受体 (GPER)/GPR30 在 MCF7 乳腺癌细胞中与 β1-肾上腺素能受体、膜相关鸟苷酸激酶 (MAGUK) 支架蛋白和蛋白激酶 A 锚定蛋白 (AKAP) 5 形成复合物
G 蛋白偶联受体 30 (GPR30),也称为 G 蛋白偶联雌激素受体 (GPER),β1-肾上腺素能受体 (β1AR) 是与乳腺癌进展有关的 G 蛋白偶联受体 (GPCR)。两种受体的 C 端尾部都包含 PSD-95/Discs-large/ZO-1 同源 (PDZ) 基序,它们通过这些基序在质膜中与膜相关鸟苷酸激酶 (MAGUK) 支架蛋白相互作用,进而与蛋白激酶 A 锚定蛋白 (AKAP) 相互作用 5。GPR30 组成型和 PDZ 依赖性地抑制 β1AR 介导的 cAMP 产生。我们假设这种抑制是这些受体的质膜复合物的结果。使用免疫共沉淀、共聚焦免疫荧光显微镜和生物发光共振能量转移 (BRET),我们表明当在 HEK293 中瞬时表达时,GPR30 和 β1AR 位于质膜复合物中。删除 GPR30 C 端 PDZ 基序 (-SSAV) 不会干扰受体复合物,表明该复合物不依赖于 PDZ。MCF7 乳腺癌细胞在质膜中表达 GPR30、β1AR、MAGUKs 和 AKAP5,免疫共沉淀显示这些蛋白质在天然条件下也非常接近地存在。此外,GPR30 在 MCF7 细胞中的表达组成型和 PDZ 依赖性地抑制 β1AR 介导的 cAMP 产生。AKAP5 还抑制 β1AR 介导的 cAMP 产生,这与 GPR30 促进的抑制不相加。这些结果表明,GPR30 和 β1AR 在 MCF7 细胞中形成不依赖 PDZ 的复合物,GPR30 通过与 MAGUK 和 AKAP5 的受体相互作用组成型和 PDZ 依赖性地抑制 β1AR 信号传导。































 京公网安备 11010802027423号
京公网安备 11010802027423号