当前位置:
X-MOL 学术
›
Battery Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strain-rich high-entropy perovskite oxide of (La0.8Sr0.2)(Mn0.2Fe0.2Cr0.2Co0.2Ni0.2)O3 for durable and effective catalysis of oxygen redox reactions in lithium-oxygen battery
Battery Energy ( IF 9.0 ) Pub Date : 2024-01-08 , DOI: 10.1002/bte2.20230053 Zhanpeng Liu 1 , Haoyang Xu 1 , Xinxiang Wang 1 , Guilei Tian 1 , Dayue Du 1 , Chaozhu Shu 1
Battery Energy ( IF 9.0 ) Pub Date : 2024-01-08 , DOI: 10.1002/bte2.20230053 Zhanpeng Liu 1 , Haoyang Xu 1 , Xinxiang Wang 1 , Guilei Tian 1 , Dayue Du 1 , Chaozhu Shu 1
Affiliation
|
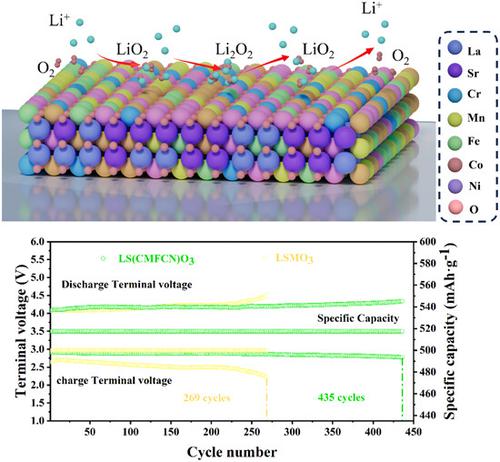
Despite their great promise as high-energy-density alternatives to Li-ion batteries, the extensive use of lithium-oxygen (Li-O2) batteries is constrained by the slow kinetics of both the oxygen evolution reaction and oxygen reduction reaction. To increase the overall performance of Li-O2 batteries, it is essential to increase the efficiency of oxygen electrode reactions by constructing effective electrocatalysts. As a high-efficiency catalyst for Li-O2 batteries, high entropy perovskite oxide (La0.8Sr0.2)(Mn0.2Fe0.2Cr0.2Co0.2Ni0.2)O3 (referred to as LS(MFCCN)O3) is designed and investigated in this article. The introduction of dissimilar metals in LS(MFCCN)O3 has the potential to cause lattice deformation, thereby enhancing electron transfer between transition metal ions and facilitating the formation of numerous oxygen vacancies. This feature is advantageous for the reversible production and breakdown of discharge product Li2O2. Consequently, the Li-O2 battery utilizing LS(MFCCN)O3 as a catalyst achieves an impressive discharge capacity of 17,078.2 mAh g−1 and exhibits an extended cycling life of 435 cycles. This study offers a useful method for adjusting the catalytic performance of perovskite oxides toward oxygen redox reactions in Li-O2 batteries.
中文翻译:

(La0.8Sr0.2)(Mn0.2Fe0.2Cr0.2Co0.2Ni0.2)O3 的富应变高熵钙钛矿氧化物可持久有效地催化锂氧电池中的氧氧化还原反应
尽管作为锂离子电池的高能量密度替代品前景广阔,但锂氧(Li-O 2)电池的广泛使用受到析氧反应和氧还原反应动力学缓慢的限制。为了提高Li-O 2电池的整体性能,必须通过构建有效的电催化剂来提高氧电极反应的效率。作为Li-O 2电池高效催化剂,设计了高熵钙钛矿氧化物(La 0.8 Sr 0.2 )(Mn 0.2 Fe 0.2 Cr 0.2 Co 0.2 Ni 0.2 )O 3 (简称LS(MFCCN)O 3 )并在本文中进行了调查。在LS(MFCCN)O 3中引入异种金属有可能引起晶格变形,从而增强过渡金属离子之间的电子转移并促进大量氧空位的形成。该特征有利于放电产物Li 2 O 2的可逆产生和分解。因此,利用LS(MFCCN)O 3作为催化剂的Li-O 2电池实现了17,078.2 mAh g -1的令人印象深刻的放电容量,并表现出435次循环的延长循环寿命。这项研究为调整钙钛矿氧化物对Li-O 2电池中氧氧化还原反应的催化性能提供了一种有用的方法。
更新日期:2024-01-08
中文翻译:

(La0.8Sr0.2)(Mn0.2Fe0.2Cr0.2Co0.2Ni0.2)O3 的富应变高熵钙钛矿氧化物可持久有效地催化锂氧电池中的氧氧化还原反应
尽管作为锂离子电池的高能量密度替代品前景广阔,但锂氧(Li-O 2)电池的广泛使用受到析氧反应和氧还原反应动力学缓慢的限制。为了提高Li-O 2电池的整体性能,必须通过构建有效的电催化剂来提高氧电极反应的效率。作为Li-O 2电池高效催化剂,设计了高熵钙钛矿氧化物(La 0.8 Sr 0.2 )(Mn 0.2 Fe 0.2 Cr 0.2 Co 0.2 Ni 0.2 )O 3 (简称LS(MFCCN)O 3 )并在本文中进行了调查。在LS(MFCCN)O 3中引入异种金属有可能引起晶格变形,从而增强过渡金属离子之间的电子转移并促进大量氧空位的形成。该特征有利于放电产物Li 2 O 2的可逆产生和分解。因此,利用LS(MFCCN)O 3作为催化剂的Li-O 2电池实现了17,078.2 mAh g -1的令人印象深刻的放电容量,并表现出435次循环的延长循环寿命。这项研究为调整钙钛矿氧化物对Li-O 2电池中氧氧化还原反应的催化性能提供了一种有用的方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号