当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid Access to Densely Functionalized Cyclopentenyl Sulfoximines through a Sc-Catalyzed Aza-Piancatelli Reaction
Organic Letters ( IF 4.9 ) Pub Date : 2024-01-08 , DOI: 10.1021/acs.orglett.3c04095 Emilie Werner 1 , Milena Wiegand 1 , Joseph Moran 1, 2, 3 , David Lebœuf 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-01-08 , DOI: 10.1021/acs.orglett.3c04095 Emilie Werner 1 , Milena Wiegand 1 , Joseph Moran 1, 2, 3 , David Lebœuf 1
Affiliation
|
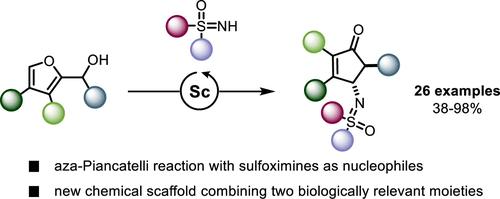
Sulfoximines make up a class of compounds of growing interest for crop science and medicinal chemistry, but methods for directly incorporating them into complex molecular scaffolds are lacking. Here we report a scandium-catalyzed variant of the aza-Piancatelli cyclization that can directly incorporate sulfoximines as nucleophiles rather than the classical aniline substrates. Starting from 2-furylcarbinols and sulfoximines, the reaction provides direct access to 4-sulfoximinocyclopentenones, a new scaffold bearing cyclopentenone and sulfoximine motifs, both of interest for bioactive compounds.
中文翻译:

通过 Sc 催化的 Aza-Piancatelli 反应快速获得稠密功能化的环戊烯亚磺酰亚胺
亚磺酰亚胺是作物科学和药物化学领域越来越感兴趣的一类化合物,但缺乏将它们直接整合到复杂分子支架中的方法。在这里,我们报道了一种钪催化的氮杂-Piancatelli环化变体,它可以直接将亚砜亚胺作为亲核试剂而不是经典的苯胺底物掺入。从 2-呋喃基甲醇和亚砜亚胺开始,该反应可直接获得 4-亚磺酰亚氨基环戊烯酮,这是一种带有环戊烯酮和亚砜亚胺基序的新支架,这两种基序都是生物活性化合物所感兴趣的。
更新日期:2024-01-08
中文翻译:

通过 Sc 催化的 Aza-Piancatelli 反应快速获得稠密功能化的环戊烯亚磺酰亚胺
亚磺酰亚胺是作物科学和药物化学领域越来越感兴趣的一类化合物,但缺乏将它们直接整合到复杂分子支架中的方法。在这里,我们报道了一种钪催化的氮杂-Piancatelli环化变体,它可以直接将亚砜亚胺作为亲核试剂而不是经典的苯胺底物掺入。从 2-呋喃基甲醇和亚砜亚胺开始,该反应可直接获得 4-亚磺酰亚氨基环戊烯酮,这是一种带有环戊烯酮和亚砜亚胺基序的新支架,这两种基序都是生物活性化合物所感兴趣的。































 京公网安备 11010802027423号
京公网安备 11010802027423号