当前位置:
X-MOL 学术
›
ACS Mater. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bisphosphonate-Based Hydrogel with pH-Responsive Minocycline Release Inhibits Microglia/Macrophages of M1 Polarization for Spinal Cord Injury Therapy
ACS Materials Letters ( IF 9.6 ) Pub Date : 2024-01-08 , DOI: 10.1021/acsmaterialslett.3c01126 Ya Li 1 , Ziqiang Wang 1 , Shan Pei 1 , Ranxi Chen 1 , Yajun Li 1 , Yuyun Liang 1 , Can Zhang 1 , Lili Wang 2 , Jianwu Dai 1, 3 , Liyang Shi 1
ACS Materials Letters ( IF 9.6 ) Pub Date : 2024-01-08 , DOI: 10.1021/acsmaterialslett.3c01126 Ya Li 1 , Ziqiang Wang 1 , Shan Pei 1 , Ranxi Chen 1 , Yajun Li 1 , Yuyun Liang 1 , Can Zhang 1 , Lili Wang 2 , Jianwu Dai 1, 3 , Liyang Shi 1
Affiliation
|
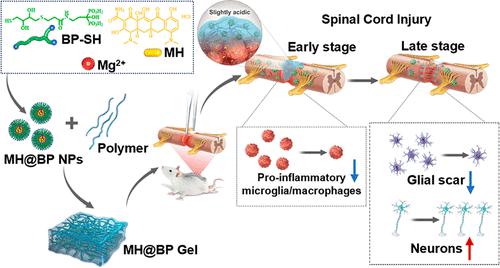
After spinal cord injury (SCI), the persistent presence of pro-inflammatory microglia/macrophages (M1 phenotype) in injury lesion is one of the major reasons for inducing neuron damage and preventing neurological function recovery. Inhibition of microglia/macrophages of M1 polarization using minocycline-based biomaterials efficiently reduces SCI of pro-inflammatory microenvironment. However, previously reported minocycline-loading biomaterials lack precise injured-microenvironment-responsive drug release behavior. Here the bisphosphonate (BP)–metal coordination method together with polymeric networks is used to deliver minocycline locally in SCI lesions, where the slight acid in the injured site triggers minocycline release. The data from in vitro cellular experiments and in vivo rat SCI model demonstrated that minocycline-loaded BP-based hydrogel (MH@BP Gel) availably inhibits microglia/macrophage polarization toward the M1 phenotype. Moreover, MH@BP Gel promotes animal motor functional recovery, inhibits glial scar formation, and promotes neurofilament- and class III β-tubulin-positive neuron survival and regeneration in vivo. Therefore, our presented MH@BP Gel provides a facile strategy for localized minocycline delivery and a promising solution for SCI therapy.
中文翻译:

基于双膦酸盐的水凝胶与 pH 响应米诺环素释放抑制小胶质细胞/巨噬细胞的 M1 极化用于脊髓损伤治疗
脊髓损伤(SCI)后,损伤病灶中促炎性小胶质细胞/巨噬细胞(M1表型)的持续存在是诱发神经元损伤并阻碍神经功能恢复的主要原因之一。使用基于米诺环素的生物材料抑制小胶质细胞/巨噬细胞的 M1 极化,可有效减少促炎微环境的 SCI。然而,先前报道的负载米诺环素的生物材料缺乏精确的损伤微环境响应性药物释放行为。在这里,双膦酸盐(BP)-金属配位方法与聚合物网络一起用于在 SCI 损伤处局部输送米诺环素,受伤部位的微酸会触发米诺环素释放。体外细胞实验和体内大鼠 SCI 模型的数据表明,负载米诺环素的 BP 水凝胶 (MH@BP Gel) 可有效抑制小胶质细胞/巨噬细胞向 M1 表型极化。此外,MH@BP Gel 还可促进动物运动功能恢复,抑制神经胶质疤痕形成,并促进体内神经丝和 III 类 β-微管蛋白阳性神经元的存活和再生。因此,我们提出的 MH@BP 凝胶为局部米诺环素递送提供了一种简便的策略,并为 SCI 治疗提供了一种有前景的解决方案。
更新日期:2024-01-08
中文翻译:

基于双膦酸盐的水凝胶与 pH 响应米诺环素释放抑制小胶质细胞/巨噬细胞的 M1 极化用于脊髓损伤治疗
脊髓损伤(SCI)后,损伤病灶中促炎性小胶质细胞/巨噬细胞(M1表型)的持续存在是诱发神经元损伤并阻碍神经功能恢复的主要原因之一。使用基于米诺环素的生物材料抑制小胶质细胞/巨噬细胞的 M1 极化,可有效减少促炎微环境的 SCI。然而,先前报道的负载米诺环素的生物材料缺乏精确的损伤微环境响应性药物释放行为。在这里,双膦酸盐(BP)-金属配位方法与聚合物网络一起用于在 SCI 损伤处局部输送米诺环素,受伤部位的微酸会触发米诺环素释放。体外细胞实验和体内大鼠 SCI 模型的数据表明,负载米诺环素的 BP 水凝胶 (MH@BP Gel) 可有效抑制小胶质细胞/巨噬细胞向 M1 表型极化。此外,MH@BP Gel 还可促进动物运动功能恢复,抑制神经胶质疤痕形成,并促进体内神经丝和 III 类 β-微管蛋白阳性神经元的存活和再生。因此,我们提出的 MH@BP 凝胶为局部米诺环素递送提供了一种简便的策略,并为 SCI 治疗提供了一种有前景的解决方案。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号