当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Disease-Associated Mutations on the Deaminase Activity of ADAR1
Biochemistry ( IF 2.9 ) Pub Date : 2024-01-08 , DOI: 10.1021/acs.biochem.3c00405 Agya Karki 1 , Kristen B Campbell 1 , Sukanya Mozumder 1, 2 , Andrew J Fisher 1, 2 , Peter A Beal 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-01-08 , DOI: 10.1021/acs.biochem.3c00405 Agya Karki 1 , Kristen B Campbell 1 , Sukanya Mozumder 1, 2 , Andrew J Fisher 1, 2 , Peter A Beal 1
Affiliation
|
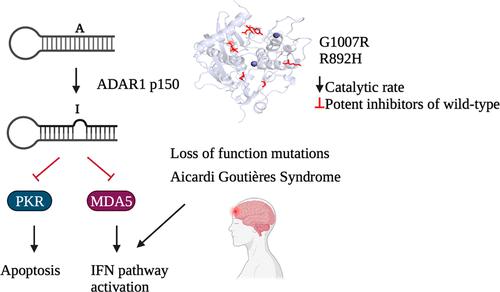
The innate immune system relies on molecular sensors to detect distinctive molecular patterns, including viral double-stranded RNA (dsRNA), which triggers responses resulting in apoptosis and immune infiltration. Adenosine Deaminases Acting on RNA (ADARs) catalyze the deamination of adenosine (A) to inosine (I), serving as a mechanism to distinguish self from non-self RNA and prevent aberrant immune activation. Loss-of-function mutations in the ADAR1 gene are one cause of Aicardi Goutières Syndrome (AGS), a severe autoimmune disorder in children. Although seven out of the eight AGS-associated mutations in ADAR1 occur within the catalytic domain of the ADAR1 protein, their specific effects on the catalysis of adenosine deamination remain poorly understood. In this study, we carried out a biochemical investigation of four AGS-causing mutations (G1007R, R892H, K999N, and Y1112F) in ADAR1 p110 and truncated variants. These studies included adenosine deamination rate measurements with two different RNA substrates derived from human transcripts known to be edited by ADAR1 p110 (glioma-associated oncogene homologue 1 (hGli1), 5-hydroxytryptamine receptor 2C (5-HT2cR)). Our results indicate that AGS-associated mutations at two amino acid positions directly involved in stabilizing the base-flipped conformation of the ADAR-RNA complex (G1007R and R892H) had the most detrimental impact on catalysis. The K999N mutation, positioned near the RNA binding interface, altered catalysis contextually. Finally, the Y1112F mutation had small effects in each of the assays described here. These findings shed light on the differential effects of disease-associated mutations on adenosine deamination by ADAR1, thereby advancing our structural and functional understanding of ADAR1-mediated RNA editing.
中文翻译:

疾病相关突变对 ADAR1 脱氨酶活性的影响
先天免疫系统依靠分子传感器来检测独特的分子模式,包括病毒双链 RNA (dsRNA),它触发反应,导致细胞凋亡和免疫浸润。作用于 RNA 的腺苷脱氨酶 (ADARs) 催化腺苷 (A) 脱氨为肌苷 (I),作为区分自身和非自身 RNA 并防止异常免疫激活的机制。ADAR1 基因的功能丧失突变是导致 Aicardi Goutières 综合征 (AGS) 的原因之一,AGS 是一种严重的儿童自身免疫性疾病。尽管 ADAR1 的 8 个 AGS 相关突变中有 7 个发生在 ADAR1 蛋白的催化结构域内,但它们对腺苷脱氨催化的特异性作用仍然知之甚少。在这项研究中,我们对 ADAR1 p110 和截短变体中的四种导致 AGS 的突变 (G1007R、R892H、K999N 和 Y1112F) 进行了生化研究。这些研究包括使用两种不同的 RNA 底物测量腺苷脱氨速率,这些底物来源于已知由 ADAR1 p110 编辑的人类转录本 (胶质瘤相关癌基因同源物 1 (hGli1),5-羟色胺受体 2C (5-HT2cR))。我们的结果表明,直接参与稳定 ADAR-RNA 复合物 (G1007R 和 R892H) 碱基翻转构象的两个氨基酸位置的 AGS 相关突变对催化的影响最为不利。位于 RNA 结合界面附近的 K999N 突变在上下文中改变了催化作用。最后,Y1112F 突变在此处描述的每种测定中都有很小的影响。 这些发现揭示了疾病相关突变对 ADAR1 腺苷脱氨的不同影响,从而促进了我们对 ADAR1 介导的 RNA 编辑的结构和功能理解。
更新日期:2024-01-08
中文翻译:

疾病相关突变对 ADAR1 脱氨酶活性的影响
先天免疫系统依靠分子传感器来检测独特的分子模式,包括病毒双链 RNA (dsRNA),它触发反应,导致细胞凋亡和免疫浸润。作用于 RNA 的腺苷脱氨酶 (ADARs) 催化腺苷 (A) 脱氨为肌苷 (I),作为区分自身和非自身 RNA 并防止异常免疫激活的机制。ADAR1 基因的功能丧失突变是导致 Aicardi Goutières 综合征 (AGS) 的原因之一,AGS 是一种严重的儿童自身免疫性疾病。尽管 ADAR1 的 8 个 AGS 相关突变中有 7 个发生在 ADAR1 蛋白的催化结构域内,但它们对腺苷脱氨催化的特异性作用仍然知之甚少。在这项研究中,我们对 ADAR1 p110 和截短变体中的四种导致 AGS 的突变 (G1007R、R892H、K999N 和 Y1112F) 进行了生化研究。这些研究包括使用两种不同的 RNA 底物测量腺苷脱氨速率,这些底物来源于已知由 ADAR1 p110 编辑的人类转录本 (胶质瘤相关癌基因同源物 1 (hGli1),5-羟色胺受体 2C (5-HT2cR))。我们的结果表明,直接参与稳定 ADAR-RNA 复合物 (G1007R 和 R892H) 碱基翻转构象的两个氨基酸位置的 AGS 相关突变对催化的影响最为不利。位于 RNA 结合界面附近的 K999N 突变在上下文中改变了催化作用。最后,Y1112F 突变在此处描述的每种测定中都有很小的影响。 这些发现揭示了疾病相关突变对 ADAR1 腺苷脱氨的不同影响,从而促进了我们对 ADAR1 介导的 RNA 编辑的结构和功能理解。































 京公网安备 11010802027423号
京公网安备 11010802027423号