当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric synthesis of (R)-baclofen and (3S,4S)-tetflupyrolimet via “on water” organocatalytic addition reactions: a tip on catalyst screening
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2024-01-08 , DOI: 10.1039/d3ob02009f Bingfu Wang 1, 2 , Jian Liu 2 , Tianxing Li 2 , Hui Jin 2 , Lixin Zhang 1, 2
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2024-01-08 , DOI: 10.1039/d3ob02009f Bingfu Wang 1, 2 , Jian Liu 2 , Tianxing Li 2 , Hui Jin 2 , Lixin Zhang 1, 2
Affiliation
|
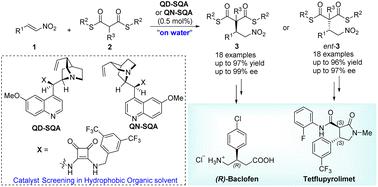
This work demonstrates asymmetric synthesis of the GABA derivative (R)-baclofen and a new herbicidal mode-of-action inhibitor (3S,4S)-tetflupyrolimet featuring low loading (0.5 mol%) organocatalytic addition reactions of dithiomalonates to nitrostyrenes under “on water” conditions. Importantly, we observed that increasing the hydrophobicity of the catalyst does not guarantee improved catalytic performance under “on water” conditions and the trends in the catalytic efficiency of different HBD catalysts under “on water” conditions (with hydrophobic additives) align more closely with those observed in pure hydrophobic organic solvents. These findings propose a valuable tip for screening organocatalysts in developing asymmetric hydrogen-bonding catalysis under “on water” conditions.
中文翻译:

通过“水上”有机催化加成反应不对称合成 (R)-巴氯芬和 (3S,4S)-四氟吡咯美特:催化剂筛选技巧
这项工作展示了 GABA 衍生物 ( R )-巴氯芬和一种新型除草作用模式抑制剂 ( 3S , 4S )-四氟吡咯美特的不对称合成,其特点是在“低负载量 (0.5 mol%) 下进行二硫代丙二酸酯与硝基苯乙烯的有机催化加成反应”水上”条件。重要的是,我们观察到增加催化剂的疏水性并不能保证在“水上”条件下提高催化性能,并且不同 HBD 催化剂在“水上”条件(使用疏水添加剂)下的催化效率趋势与那些更接近在纯疏水性有机溶剂中观察到。这些发现为筛选有机催化剂以开发“水上”条件下的不对称氢键催化提供了宝贵的建议。
更新日期:2024-01-12
中文翻译:

通过“水上”有机催化加成反应不对称合成 (R)-巴氯芬和 (3S,4S)-四氟吡咯美特:催化剂筛选技巧
这项工作展示了 GABA 衍生物 ( R )-巴氯芬和一种新型除草作用模式抑制剂 ( 3S , 4S )-四氟吡咯美特的不对称合成,其特点是在“低负载量 (0.5 mol%) 下进行二硫代丙二酸酯与硝基苯乙烯的有机催化加成反应”水上”条件。重要的是,我们观察到增加催化剂的疏水性并不能保证在“水上”条件下提高催化性能,并且不同 HBD 催化剂在“水上”条件(使用疏水添加剂)下的催化效率趋势与那些更接近在纯疏水性有机溶剂中观察到。这些发现为筛选有机催化剂以开发“水上”条件下的不对称氢键催化提供了宝贵的建议。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号