Journal of Chemical Sciences ( IF 1.7 ) Pub Date : 2024-01-06 , DOI: 10.1007/s12039-023-02234-6 Damir A. Safin
|
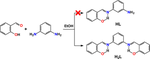
A.Z. El-Sonbati et al., in their article “Synthesis, characterization of Schiff base metal complexes and their biological investigation” (Appl. Organometal. Chem. 2019, 33, e5048) reported on the synthesis of a new Schiff base named (E)-2-(((3-aminophenyl)imino)methyl)phenol (also known as N-salicylidene-m-phenylenediamine, HL), which was obtained through condensation reaction of m-phenylenediamine and salicylaldehyde in a 1:1 molar ratio. The reported Schiff base HL was involved in the complexation reaction with a series of metal cations named Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II). Although no crystal structures of either the parent ligand HL or its complexes with the mentioned metal cations were reported, the newly synthesized compounds were characterized by means of elemental analysis, IR-, UV-vis- and 1H NMR spectroscopy, mass-spectrometry, magnetic susceptibility, conductivity and thermal analyses. The antimicrobial activity of the discussed compounds, together with the molecular docking results, were also reported. Additionally, both the Schiff base HL and its metallocomplexes were thoroughly examined by quantum chemical calculations. Despite a plethora of different methods being applied to characterize the obtained compounds, herein, I argue that discussion of the results is doubtful. Furthermore, the results of quantum chemical calculations are dubious and must be reconsidered. Although numerous synthetic attempts failed in this work, the hypothetically possible Schiff base HL was revisited using quantum chemical calculations.
Graphical abstract
A.Z. El-Sonbati et al., in their article “Synthesis, characterization of Schiff base metal complexes and their biological investigation” (Appl. Organometal. Chem. 2019, 33, e5048) reported on a new Schiff base (E)-2-(((3-aminophenyl)imino)methyl)phenol (also known as N-salicylidene-m-phenylenediamine, HL) and its metallocomplexes. Herein, I argue that the discussion of the results is doubtful.
中文翻译:

作为间苯二胺和水杨醛缩合产物的单官能化席夫碱与双官能化席夫碱:实验和计算研究
AZ El-Sonbati等人。,在他们的文章“席夫碱金属配合物的合成、表征及其生物学研究”(Appl. Organometal. Chem. 2019, 33 , e5048)中报道了一种名为 ( E )-2-((( 3-氨基苯基)亚氨基)甲基)苯酚(又称N-水杨基间苯二胺,HL ),由间苯二胺与水杨醛按1:1摩尔比缩合反应制得。报道的席夫碱HL参与与一系列金属阳离子 Cr(III)、Mn(II)、Fe(III)、Co(II)、Ni(II)、Cu(II)、Zn( II) 和镉(II)。尽管没有报道母体配体HL或其与上述金属阳离子的配合物的晶体结构,但新合成的化合物通过元素分析、IR-、UV-vis-和1 H NMR 光谱、质谱、磁化率、电导率和热分析。还报告了所讨论的化合物的抗菌活性以及分子对接结果。此外,席夫碱HL及其金属配合物均通过量子化学计算进行了彻底检查。尽管应用了大量不同的方法来表征所获得的化合物,但本文中我认为对结果的讨论是值得怀疑的。此外,量子化学计算的结果是可疑的,必须重新考虑。尽管在这项工作中多次合成尝试都失败了,但使用量子化学计算重新审视了假设可能的席夫碱HL 。
图形概要
AZ El-Sonbati等人。,在他们的文章“席夫碱金属配合物的合成、表征及其生物学研究”(Appl. Organometal. Chem. 2019, 33 , e5048)中报道了一种新的席夫碱 ( E )-2-(((3-氨基苯基)亚氨基)甲基)苯酚(也称为N-水杨基-间苯二胺,HL)及其金属配合物。在此,我认为对结果的讨论是值得怀疑的。














































 京公网安备 11010802027423号
京公网安备 11010802027423号